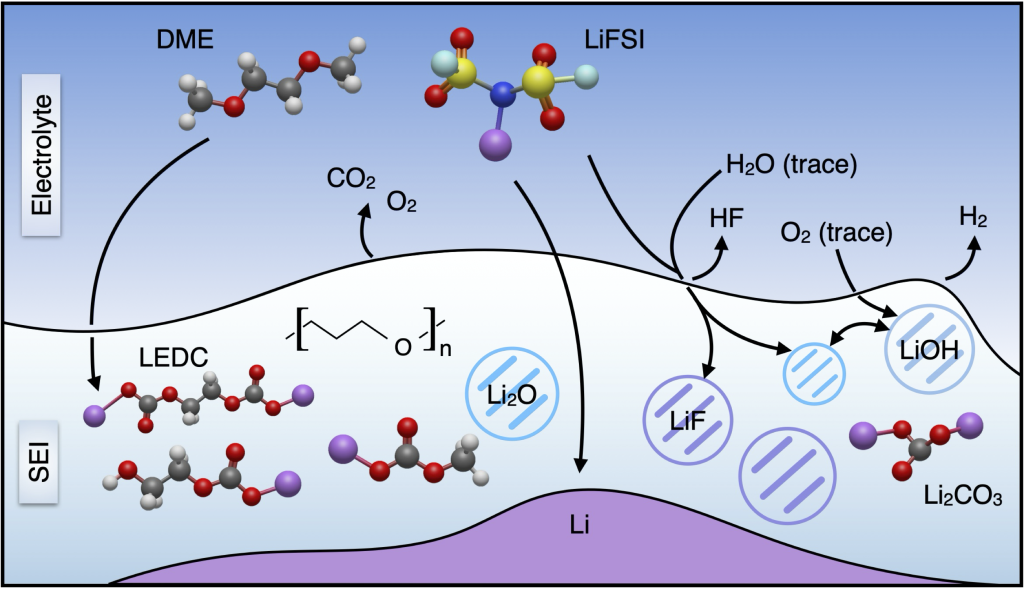
Interphases and interfaces
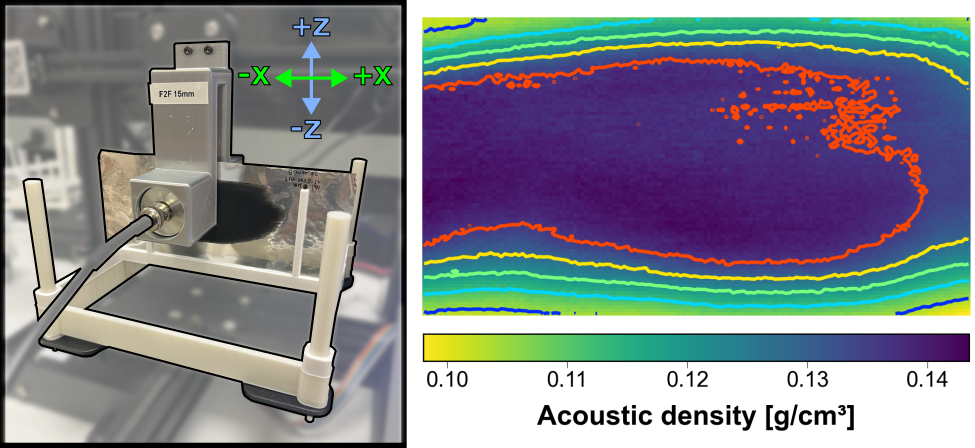
Commercial Li-ion batteries use liquid electrolytes paired with porous, solvent-processed composite electrodes. We aim to understand the electrode and electrolyte interfaces for emerging battery chemistries such as silicon anodes and solid electrolytes, and engineer interfaces that are chemo-mechanically stable under battery charge and discharge. We utilize a multi-scale approach, using techniques such as non-destructive ultrasonic characterization, to examine the relationships between individual material properties and device-level behavior.

Processing and manufacturing science
Battery materials processing has a significant effect on mechanical and electrochemical properties, including the synthesis of solid electrolytes and new cathode chemistries. We aim to explore how materials processing routes impact physical properties and electrochemical performance. Ultimately, new processing methods can lead to lower costs and improved energy efficiency. We have worked closely with industry collaborators and sponsors, such as Arkema, Ford and SES AI.

Electrochemical conversion and extraction
The rechargeable battery is a type of electrochemical cell that contributes to the decarbonization of the transportation and grid storage sectors. Electrochemical cells can also be used to power the production of chemical fuels and industrial commodities. Using renewable electricity to power manufacturing plants may reduce their energy consumption and emissions, but electrocatalytic cells have yet to undergo industrial-scale deployment. We aim to apply our understanding of reactive interfaces in electrochemical energy storage to develop new forms of electrochemical conversion, including the selective extraction of critical minerals.
