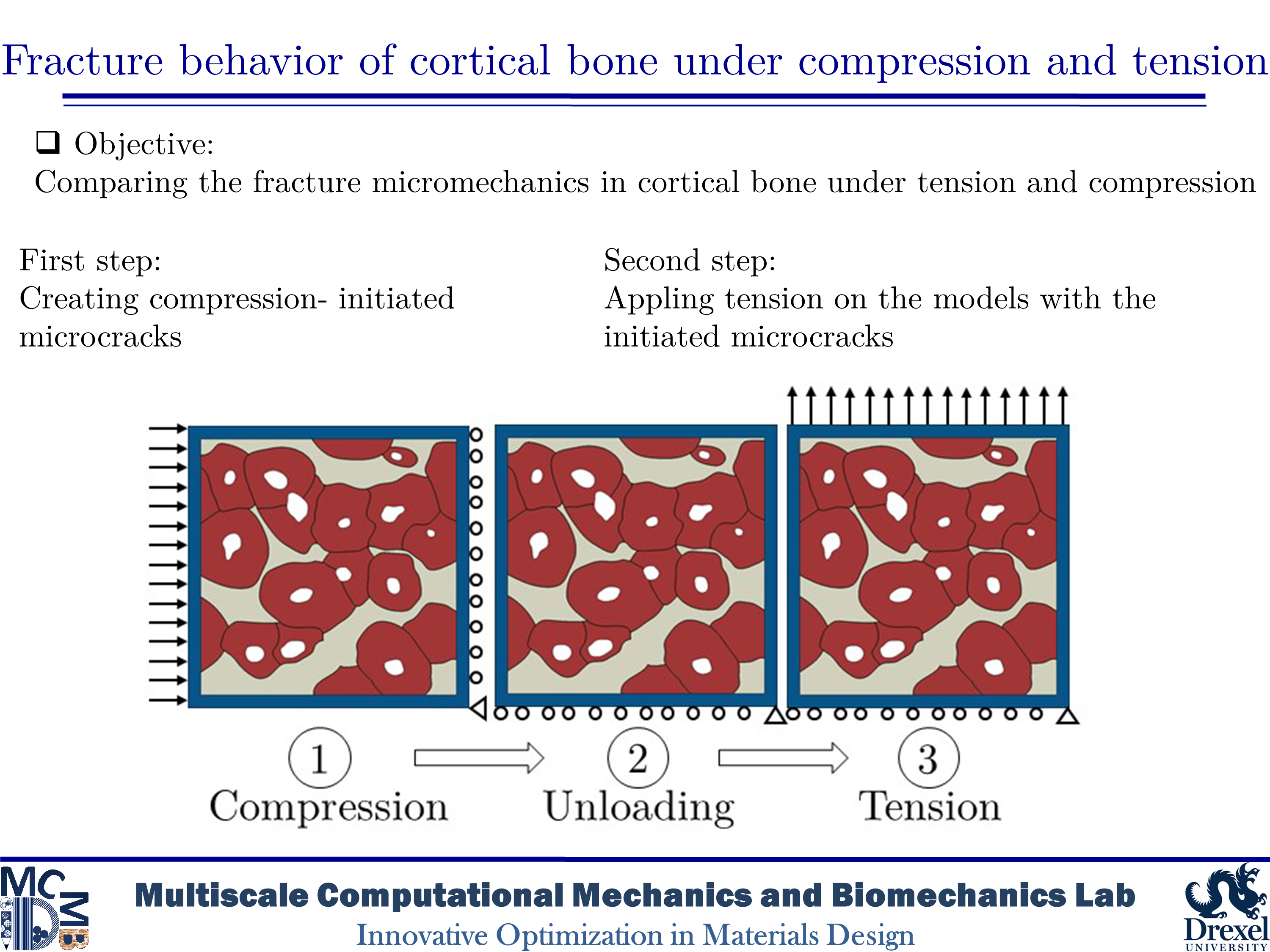
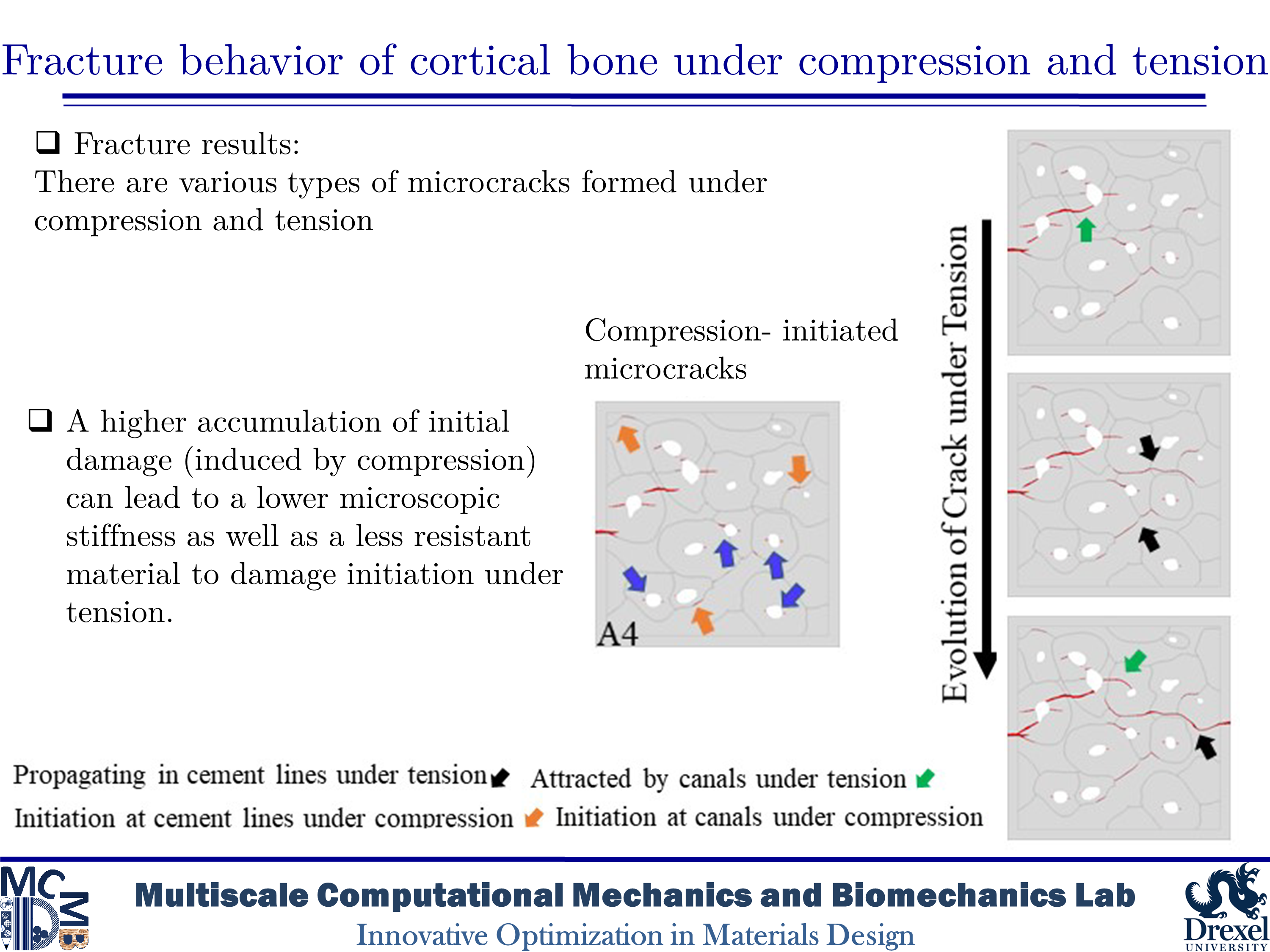
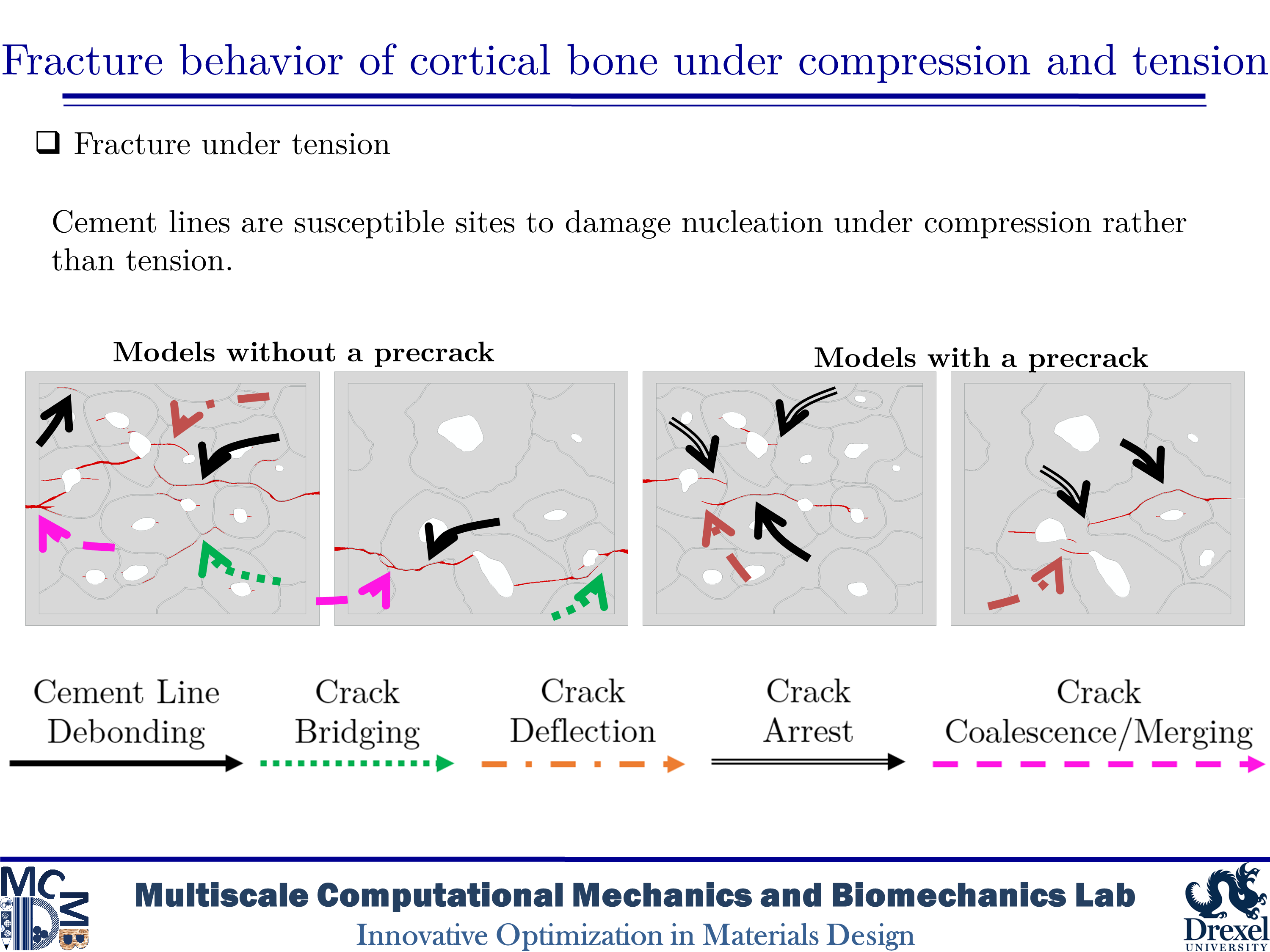
Fracture behavior of cortical bone under compression and tension
Human cortical bone has a hierarchical and anisotropic structure, resulting in its mechanical behavior and response being dependent on the type and direction of imposed loadings. Microcracks in cortical bone can form under both tensile and compressive loadings. Thus, various toughening mechanisms can be activated under different circumstances. Although there is a wide variety of research on bone fracture, there are limited in-depth studies on the influence of initial microdamage accumulation in bone on its fracture behavior. In this study, we would like to determine the impact of initial microcracking accumulation on the fracture trajectory of human microstructural cortical bone samples. Our goal is to create microdamage of varying severity to analyze how the presence of initial microcracks and how their quantity affect damage evolution and fracture trajectory.
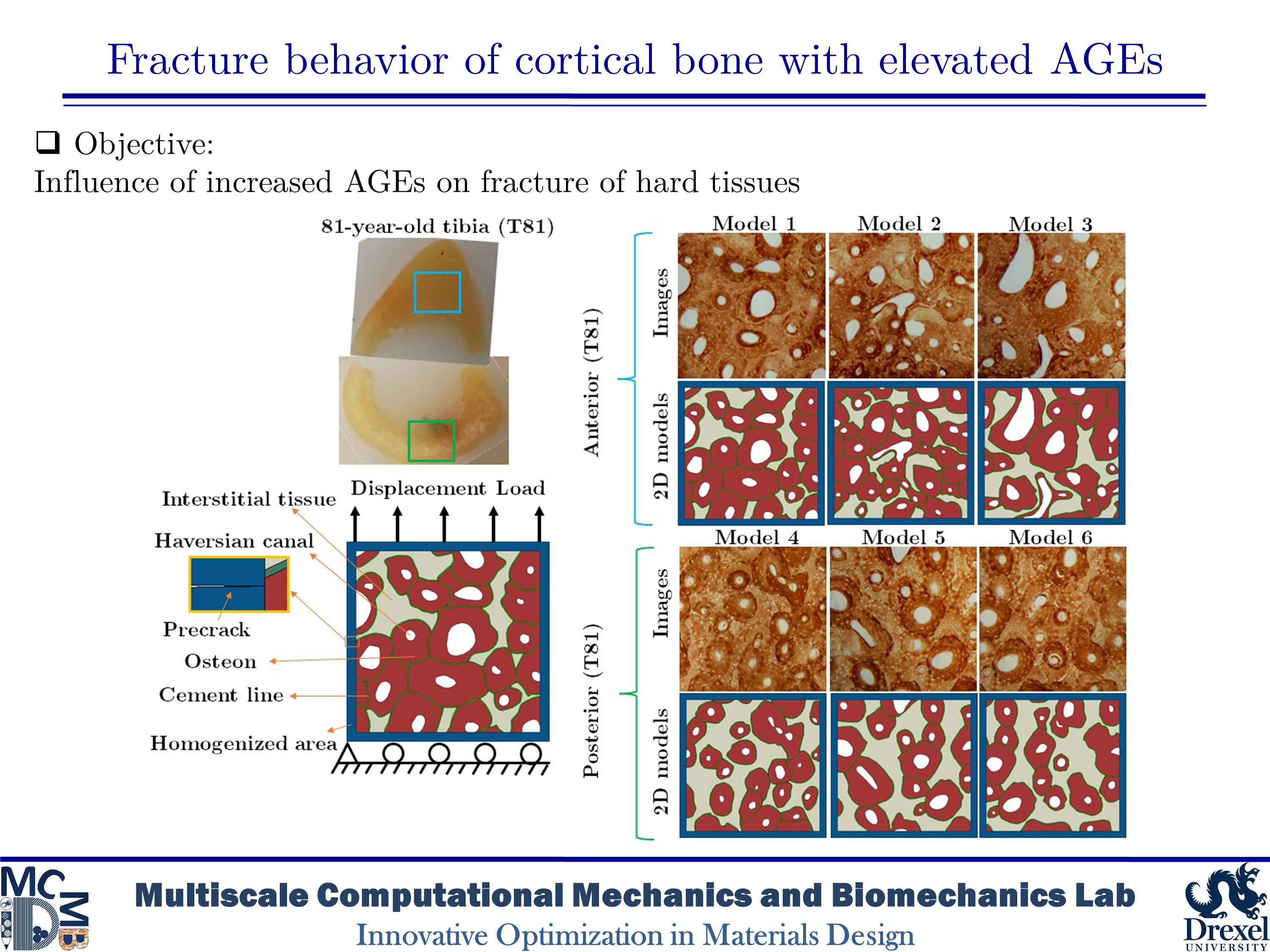
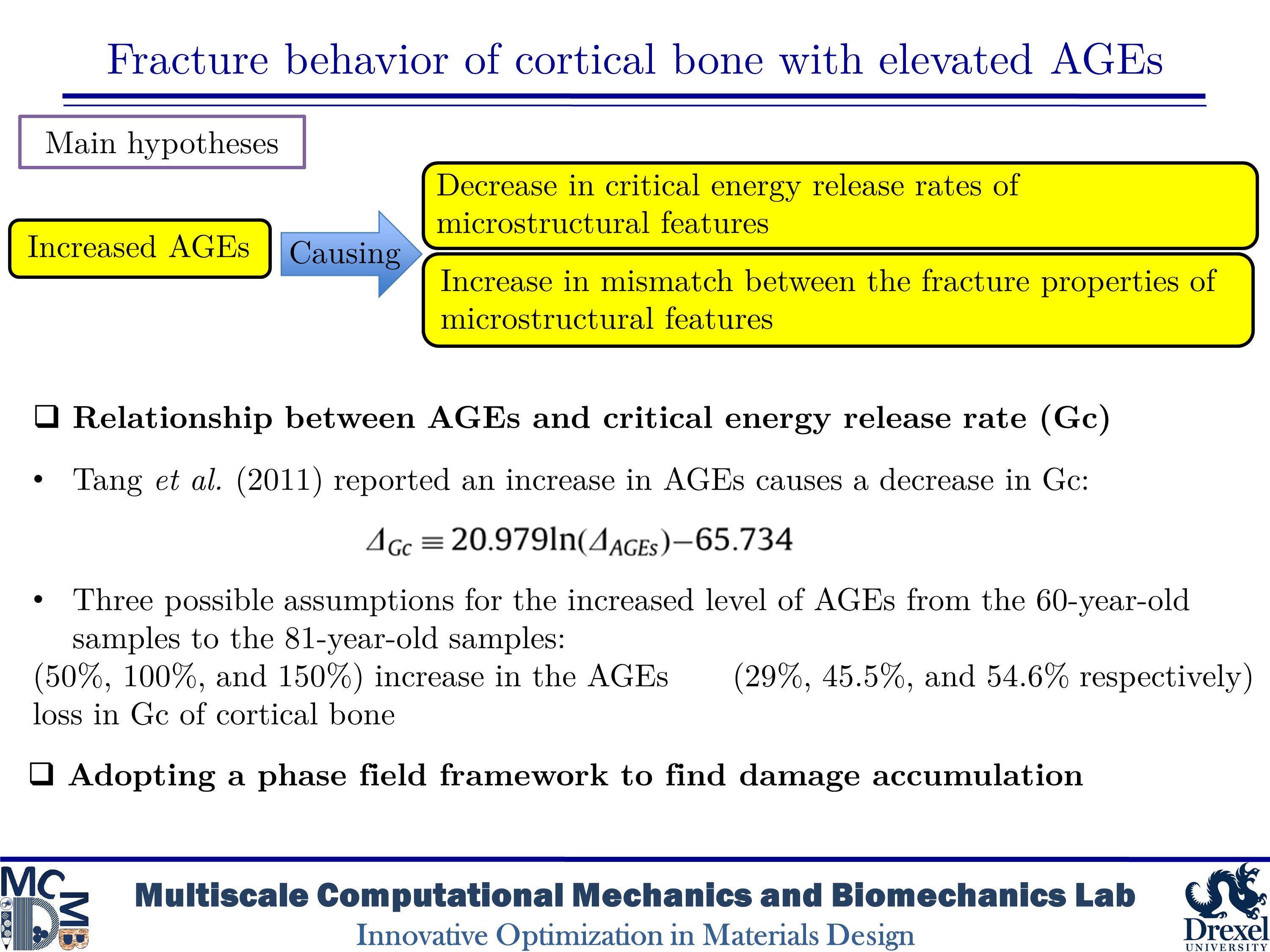
Fracture behavior of cortical bone with elevated AGEs
Diabetes and aging are associated with increased fracture risk in human bone, which impacts the quality of life and increases the medical costs especially in elderly ages. The increased fracture risk among those with type 1 diabetes (T1D) could be caused by deficiencies in the bone structure such as a decrease in the moment of inertia and the cross-sectional area of the cortical bone. However, the case of type 2 diabetes mellitus (T2D) is not typically linked to the reduction in the bone mass. In human bone with T2D, fracture resistance is affected by bone quality and changes in the organization of bone’s constituents. One of the changes related to the bone quality is the accumulation of advanced glycation end-products (AGEs) produced by non-enzymatic glycation (NEG). AGEs have impacts on bone mineralization, material properties, microstructure, and microdamage accumulation. An increase in AGEs is highly tied in the reduction in fracture resistance of the bone structure by aging. However, toughening mechanisms associated with the bone matrix diagnosed with T2D are not fully described at the microscale level. In the present study, our purpose is to evaluate the influence of AGEs on the fracture phenomena in human cortical bone microstructure.
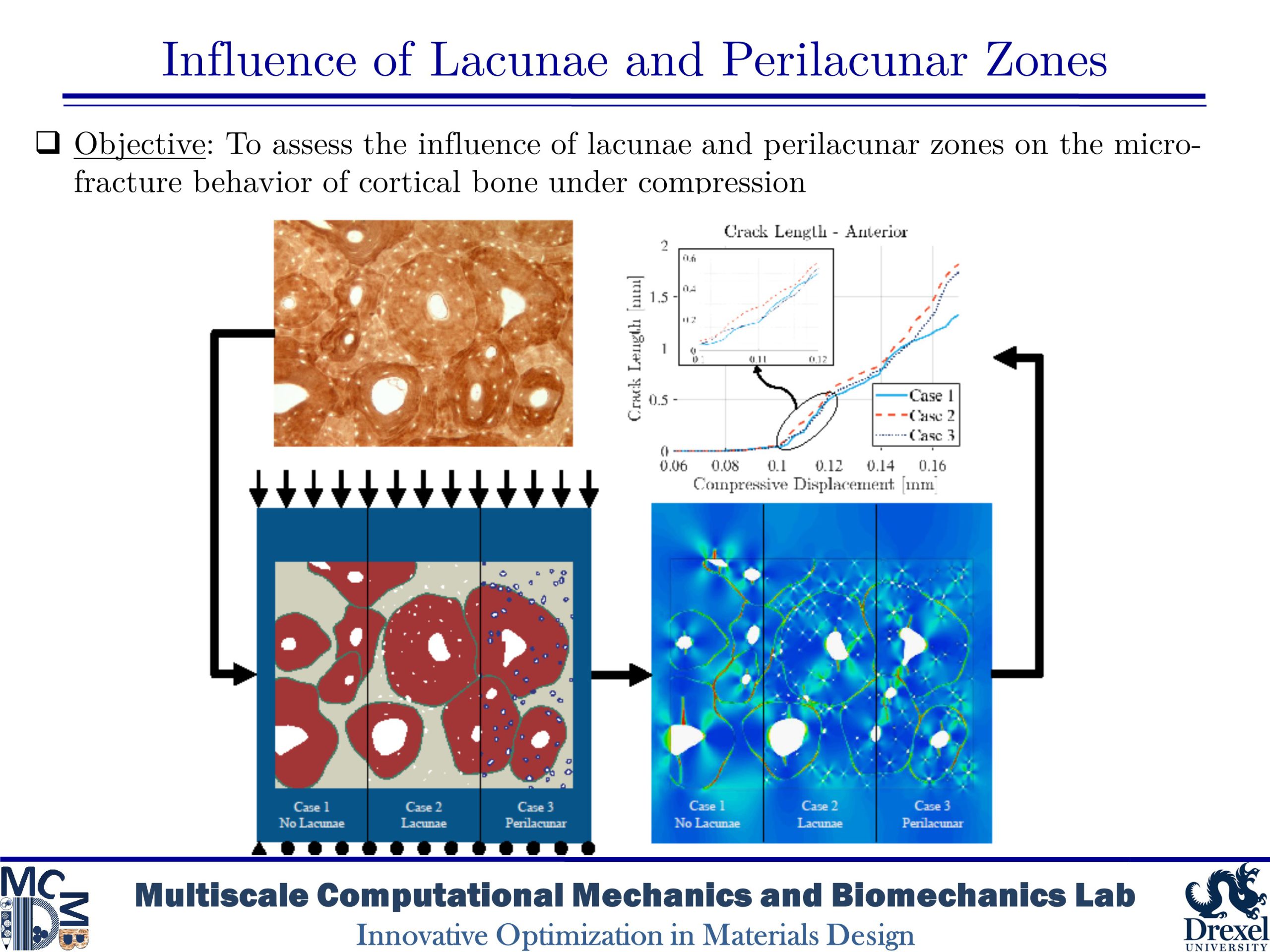
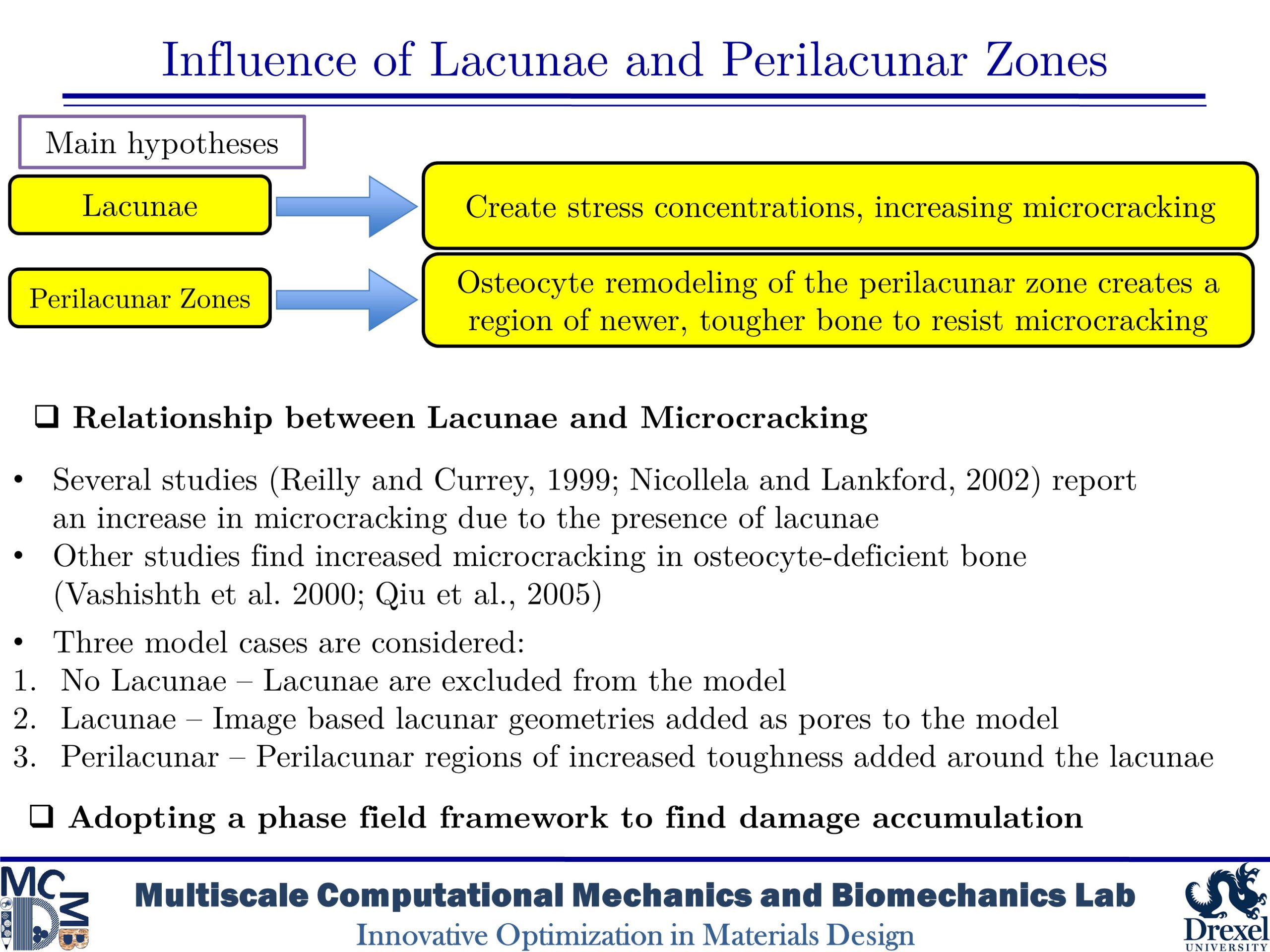
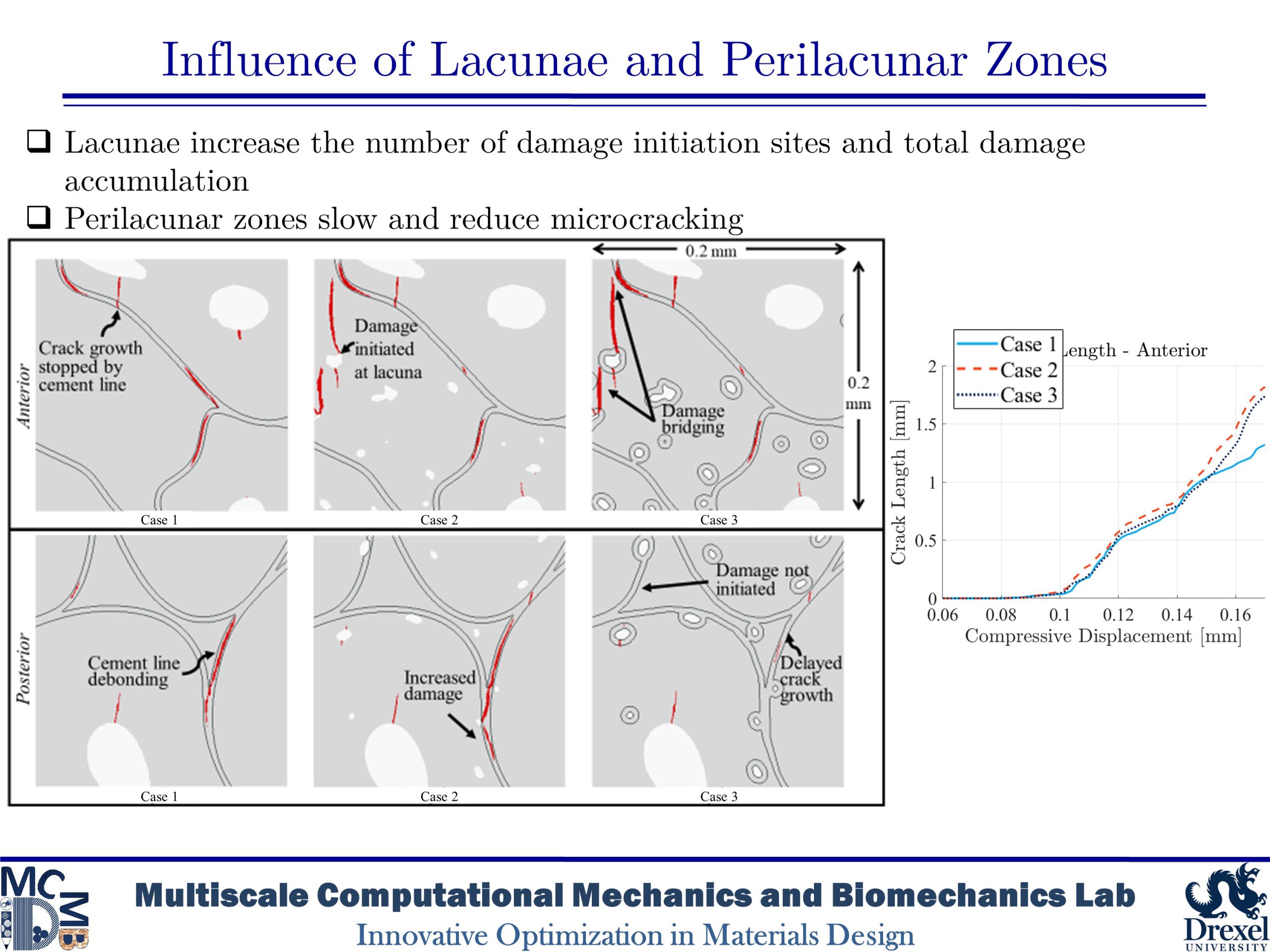
Influence of Lacunae and Perilacunar Zones
The mechanical behavior of cortical bone is influenced by microstructural components such as osteons, Haversian canals, and osteocyte lacunae that arise from biological remodeling processes. This study takes a computational approach to investigate the role of the perilacunar zones formed by the local remodeling processes of lacunar-dwelling osteocytes by utilizing phase-field finite element models based on histological imaging of human bone. The models simulated the microdamage accumulation that occurs in cortical bone under transverse compression in bone without lacunae, with lacunae, and with a perilacunar zone surrounding lacunae in order to investigate the role of these features. The results of the simulations found that while lacunae create stress concentration which initiate further damage, perilacunar regions can delay or prevent the emergence and growth of microcracks.
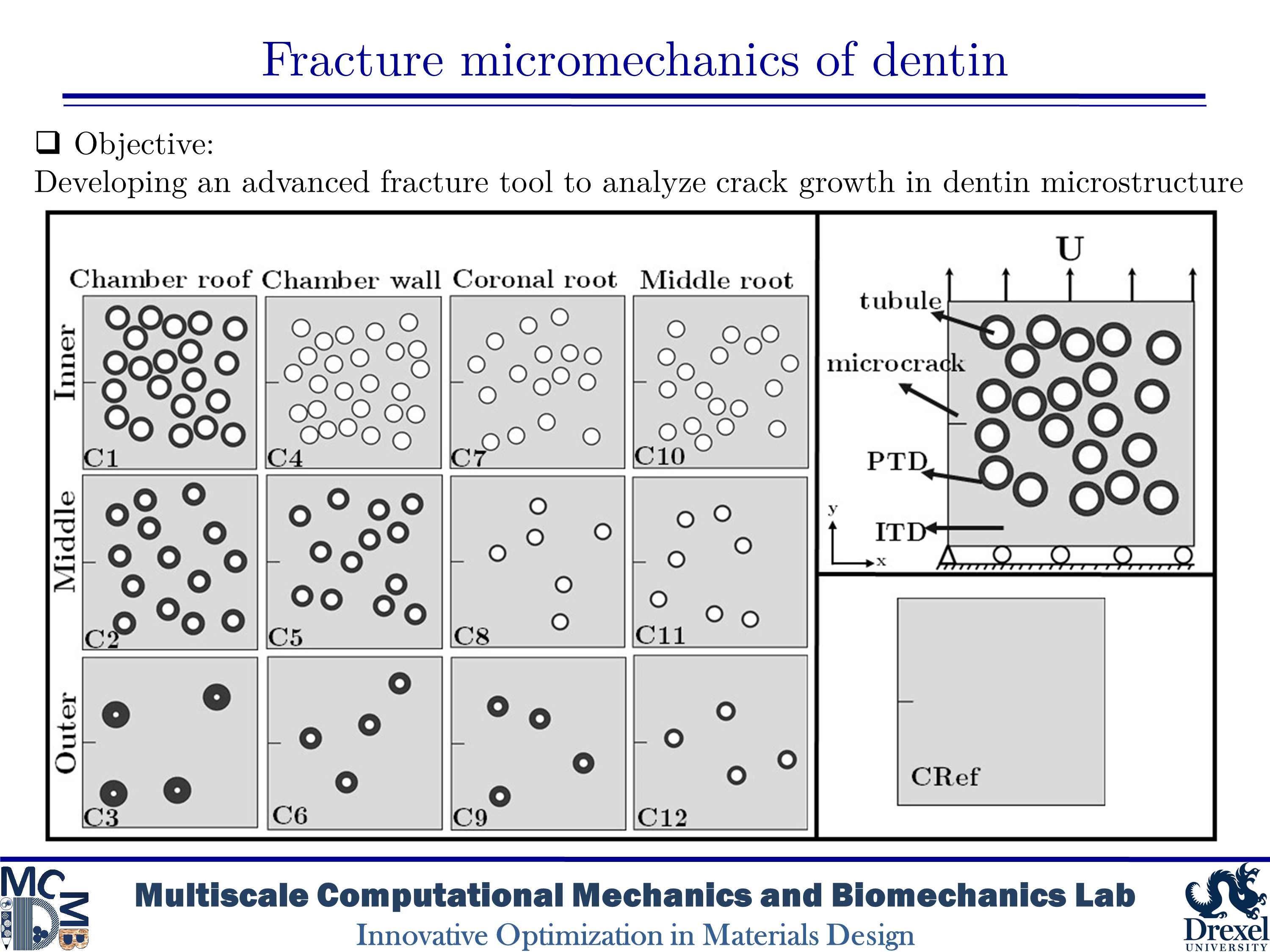
Micromechanics Fracture of Human Dentin
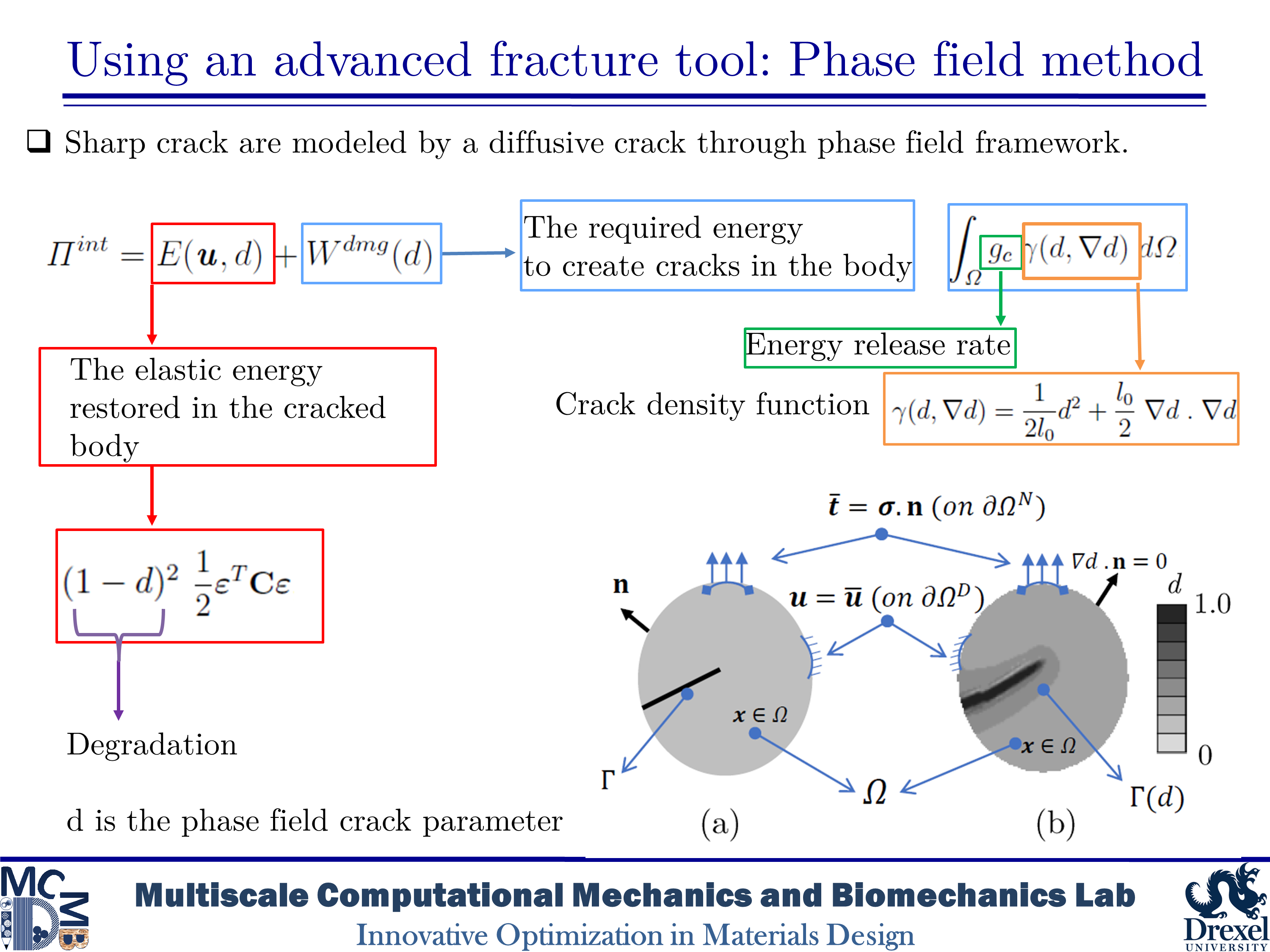
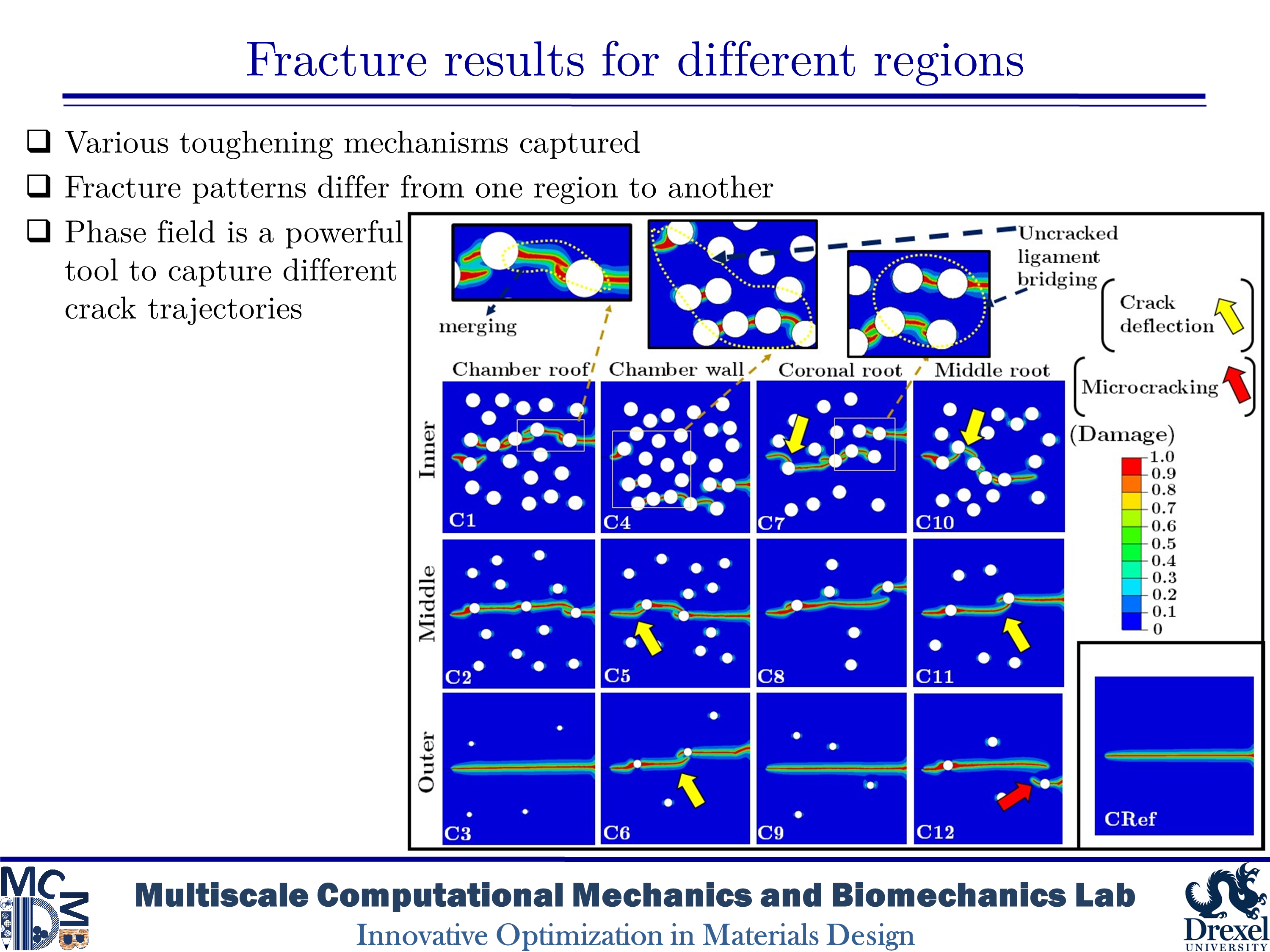
Dentin is a major structural component in human teeth. It carries and transfers the masticatory loads from the enamel surface to the jaw bone. This composite material has a unique hierarchical structure containing staggered arrays of mineral crystals embedded in a protein matrix. At the microscale level, a distinct feature is the existence of dentinal tubules. The tubules are surrounded by highly mineralized cylindrical cuffs of peritubular dentin (PTD). PTDs are embedded in a protein matrix known as intertubular dentin (ITD). The density and diameter of tubules vary from one region to another. The distinctive microstructural features have considerable effects on the mechanical and fracture resistance of dentin. Here, we are interested in studying fracture behavior in such a biological composite. An in-depth understanding of fracture micromechanisms in dentin provides valuable information to improve dental restoration techniques. In the present research, we aim to analyze crack growth trajectory in various regions of human dentin. We create 2D plain-strain models of the dentin microstructure. In order to capture various toughening mechanisms, we implement the brittle model of the phase-field method into a subroutine using Abaqus software. The results show that microcracking in PTD is found as an effective toughening mechanism in all simulations. The present findings also indicate that an increase in the density of tubules leads to an increase in the rate of crack deflection as well as a decrease in the toughness of dentin.
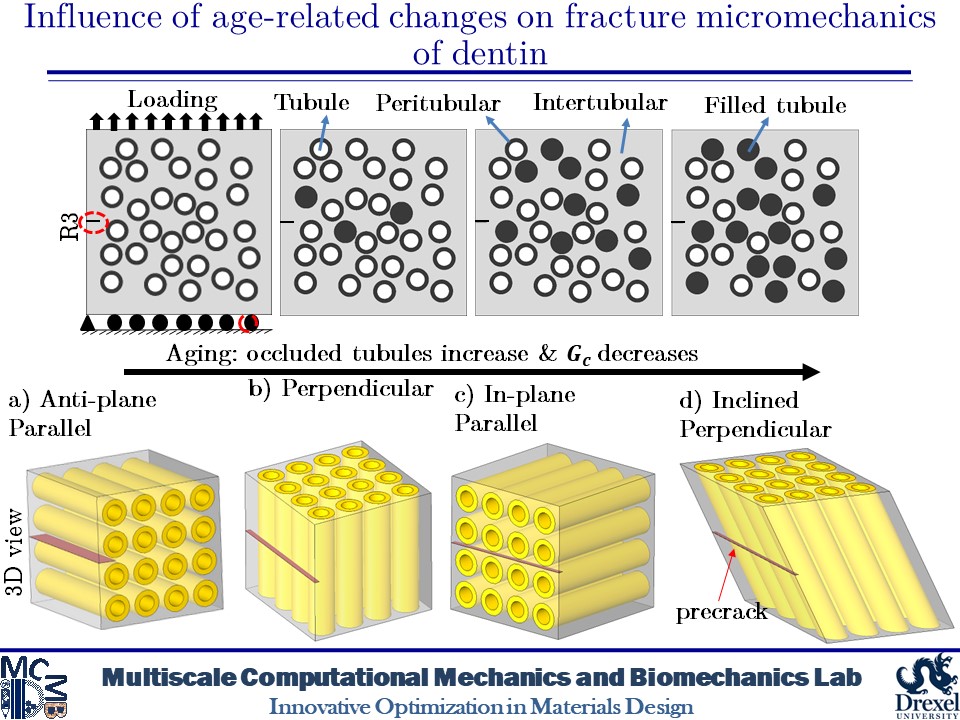
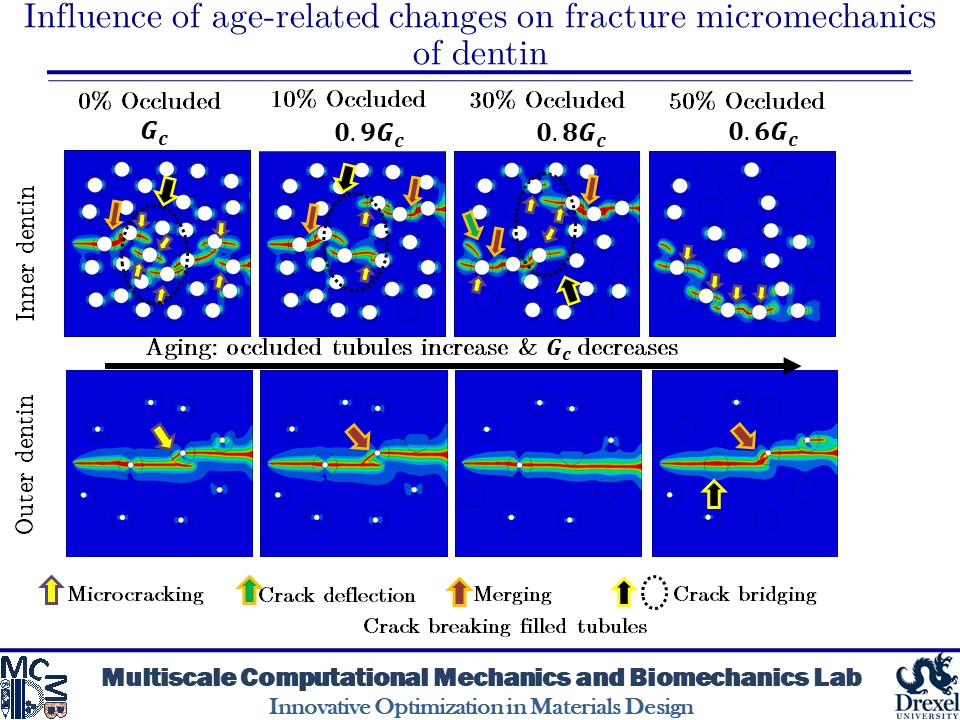
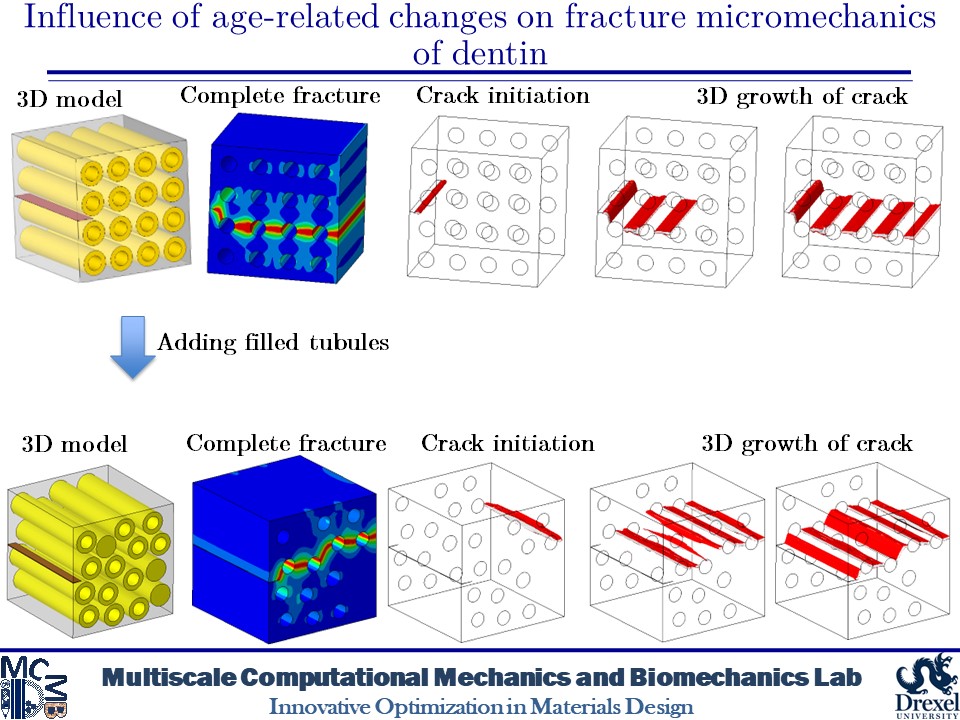
Influence of Age-related Changes on Crack Growth Trajectories and Toughening Mechanisms in Human Dentin
Dentin microstructure undergoes changes with age and its materials properties degrade over time. These changes can be increased filled tubules and decreased materials properties due to increased advanced glycation end-products (AGEs) crosslinks in dentin tissue. These alterations in the microscale can affect the fracture behavior of dentin. At the microscale level, a distinct feature is the existence of dentinal tubules. The tubules are surrounded by highly mineralized cylindrical cuffs of peritubular dentin (PTD). PTDs are embedded in a protein matrix known as intertubular dentin (ITD). These tubules can be filled with minerals over time. Here, we create 2D and 3D models of dentin microstructure with morphological data of human molars and use a phase field fracture framework to analyze crack growth trajectories. The simulations show an increase in the number of filled tubules can deactivate the toughening mechanisms such as crack deflection and microcracking. In addition, filled tubules have adverse impacts on the ability of peritubular dentin to shield microcracking. The present findings also show that an increase in the AGEs level can result in increased brittleness.
Related Publications
-
- Maghami, E., Najafi, A.R., “Influence of age-related changes on crack growth trajectories and toughening mechanisms in human dentin,” Dentin Materials, 2022.
- Maghami, E., Moore, J.P., Josephson, T.O., Najafi, A.R., “Damage analysis of human cortical bone under compressive and tensile loadings,” Computer Methods in Biomechanics and Biomedial Engineering, 2021.
- Maghami, E., Josephson, T.O., Moore, J.P., Rezaee, T., Freeman, T.A., Karim L., Najafi, A.R., “Fracture behavior of human cortical bone: Role of advanced glycation end-products and microstructural features,” Journal of Biomechanics, vol. 125 2021.
- Josephson, T.O., Moore, J.P., Maghami, E., Freeman, T.A., and Najafi, A.R., “Computational study of the mechanical influence of lacunae and perilacunar zones in cortical bone microcracking,” Journal of the Mechanical Behavior of Biomedical Materials, 126, 2022.
- Maghami, E., Pejman, R. and Najafi, A.R., “Fracture micromechanics of human dentin: A microscale numerical model,” Journal of the Mechanical Behavior of Biomedical Materials, p. 104171, 2020. https://doi.org/10.1016/j.jmbbm.2020.104171
