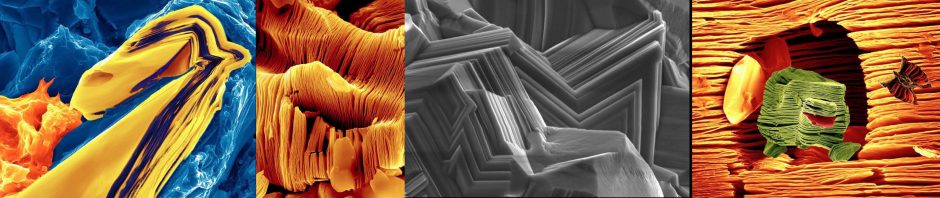
What constitutes a MAX Phase?
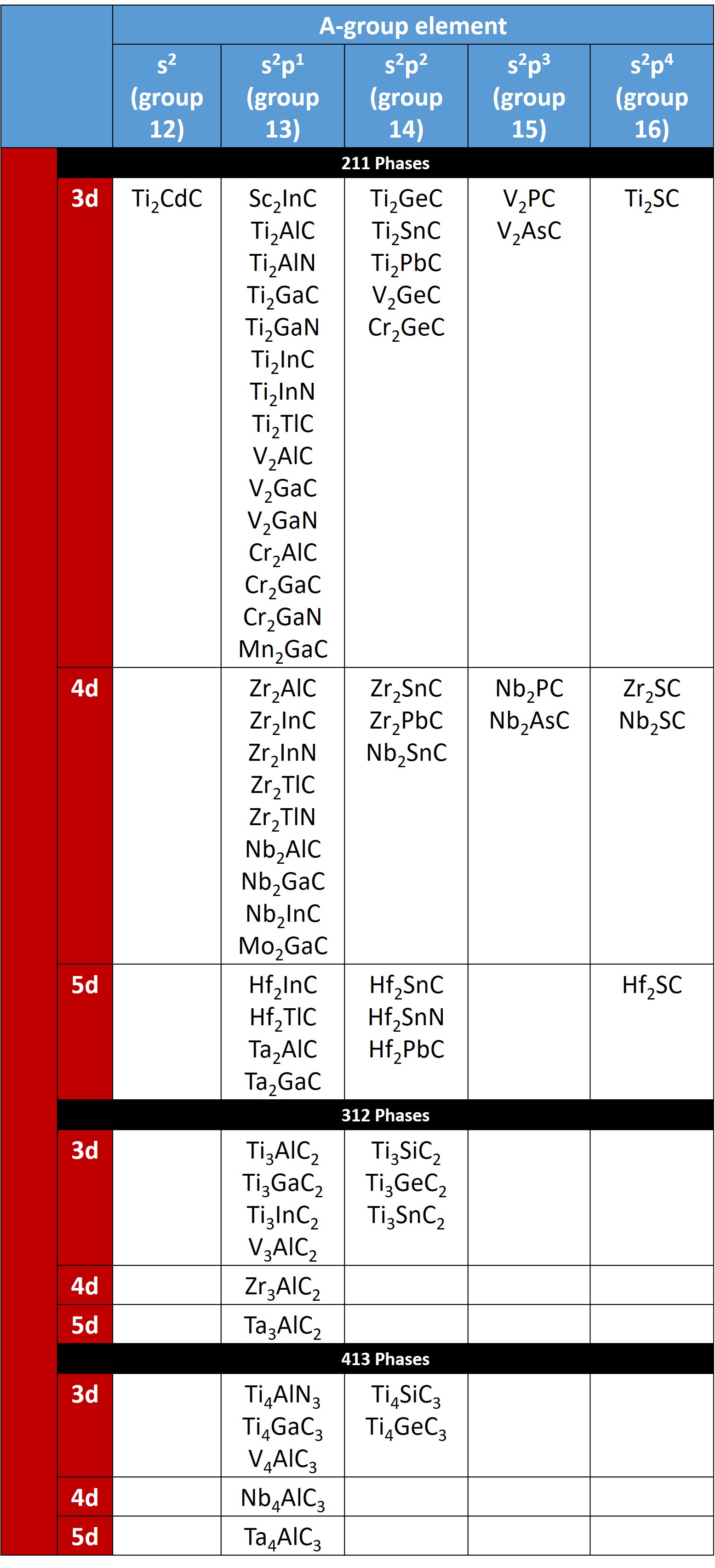
The MAX Phases form a large family of ternary carbides with the general formula Mn+1AXn,
where n = 1–3, M is an early transition metal, A is an A-group element (mostly IIIA and IVA), and X is C and/or N:

These phases are layered hexagonal compounds and belong to space group D6h 4–P63/mmc), with two formula units per unit cell. The MAX phases can be classified according to their values of n: “211” for M2AX (n=1), “312” for M3AX2 (n=2), and “413” for M4AX3 (n=3). So far, 60 MAX Phases have been discovered.
Table of the known Mn+1AXn phases:
Sorted by stoichiometry and valence electron configuration for the M and A elements. Click to see structural properties. Also see the review article by Sun et al. for more information on MAX phase structure, properties, and applications.
Why MAX Phases?
As a consequence of their layered structure, these materials kink and delaminate during deformation and also exhibit an unusual, and sometimes unique, combination of properties; they are not sure whether they want to be metals or ceramics. While they conduct heat and electricity like metals, they are elastically stiff, strong, brittle, and heat-tolerant like ceramics. They are resistant to chemical attack, readily machinable, and thermal shock, damage tolerant, and sometimes fatigue, creep, and oxidation resistant.
Current research on MAX phases is moving in many directions. Our group is working along with other research groups toward synthesis of new MAX phases in both bulk and thin film form and MAX phase composites, establishment of structure-property relationships through a wide variety of characterization techniques, and optimization for applications including those in nuclear industries and renewable energy. See our publications for more information.
For more information, go here to see comprehensive list of publications on MAX phases.