Research
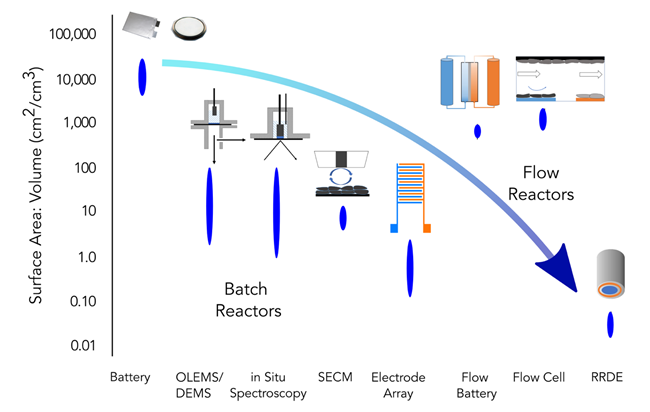
Nonaqueous battery lifetime: Nonaqueous batteries contain densely packed particles and minimal electrolyte in a sealed cell. As a chemical engineer, we recognize this as a batch-mode reactor with uncontrolled transport; it is economically necessary but extremely difficult to analyze. Accordingly, a hallmark of our battery work applies tools like the rotating-disk electrode (RDE) to deconvolute bulk transport and interfacial reaction. Drawing further inspiration from our work in electrocatalysis, we have recognized that the problem of battery lifetime is at heart a problem of catalytic selectivity. We therefore frequently implement four-electrode collector- generator experiments like the rotating ring disk electrode (RRDE) and our own novel devices to measure catalyst selectivity and the stability of intermediates. These straightforward but powerful approaches enable us to tackle both long-standing challenges in Li-ion batteries and emerging phenomena in “beyond-Li” systems. Our work in this field is supported by the National Science Foundation.
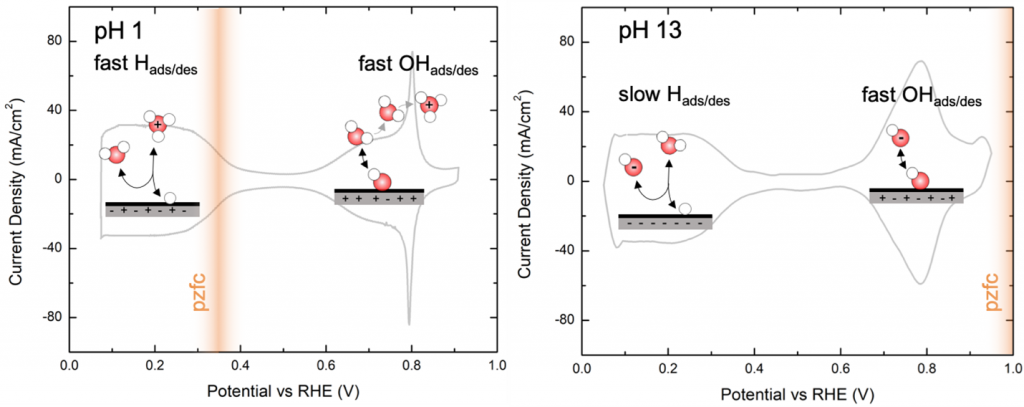
“Beyond adsorption” in hydrogen electrocatalysis: Adsorption (binding) energies on metals and oxides are known as strong indicators for catalytic performance in gases and certain liquids. However, hydrogen evolution and oxidation (HER /HOR) have exchange current densities two orders of magnitude lower in base than in acid, even though the hydrogen binding energy does not change. In collaboration with the Snyder Lab, we are combining electroanalytical and surface-science methods with detailed microkinetic models to determine the origins of pH-dependent kinetics. Our studies have shown that factors like OH binding strength increase the reaction rate by lowering the kinetic barriers, not the thermodynamic energy, for H adsorption. Experimentally quantifying the kinetic barriers to thermodynamic energy changes is rapidly emerging as the next great frontier in electrocatalysis research, and our group aims to combine tools from many disciplines to be at the forefront of this movement. Our work in this field is supported by the National Science Foundation.
Adding value with electron-holes: Many desirable electrochemical reactions are reduction processes that derive electrons from the oxygen evolution reaction (OER), a sluggish reaction with minimal product value. Replacing the OER with value-added oxidation half-reactions would vastly improve the economics of electrochemical hydrogen production, CO2 valorization, and many other processes. Our work explores potential value-added oxidation reactions to better understand their reaction mechanisms and avenues for performance improvement. These efforts are funded by the NSF and ACS Petroleum Research Fund.
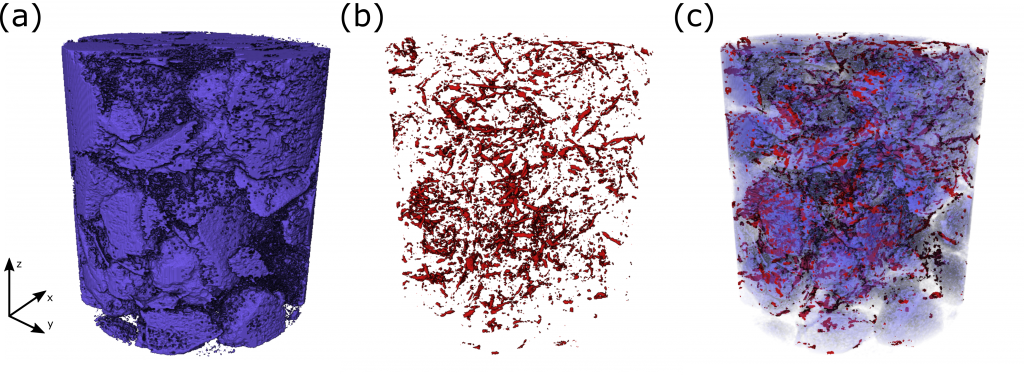
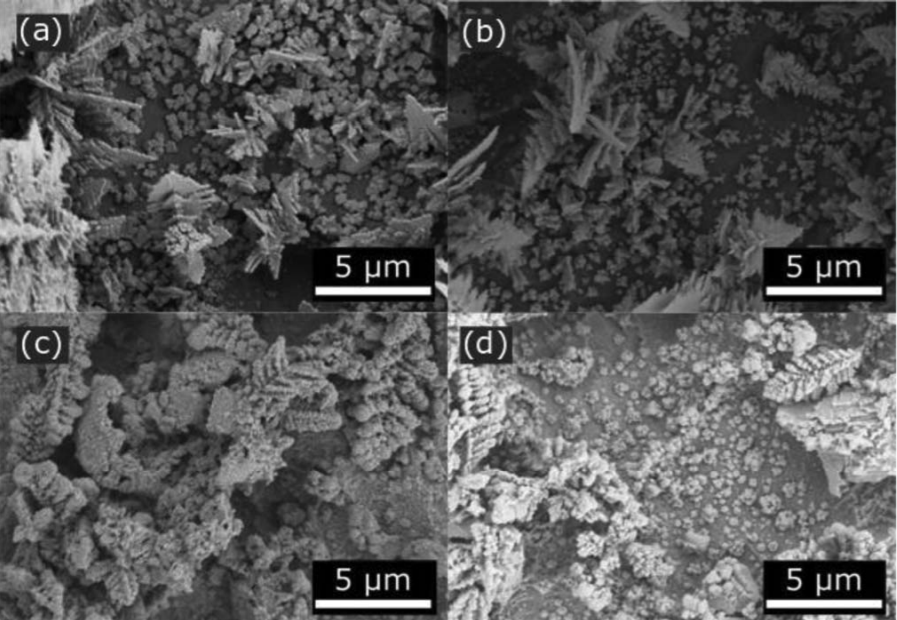
Charge transport in composite films: Our expertise in deconvoluting bulk and interfacial charge transport and reaction has developed critical insight into how material and processing parameters affect the structure and performance of composite electrodes. Despite decades of commercial relevance, there is still no universal recipe for processing a good battery (or fuel cell) electrode. This inability results in a highly empirical manufacturing paradigm in which parameters are optimized over expensive trial-and-error. Often, the most reliable guide for producing high-performance films is the intuition of an individual with decades of experience. In collaboration with the Alvarez Lab, we apply our skills to isolate transport and reaction effects to understand processing-structure-function relationships in battery electrodes. We are applying similar skills to PEM fuel cells containing novel ionomers with the Snyder Group. These efforts have been funded by DOE and NSF.
