We are interested in biomaterials-immune cell interactions, aiming to reduce immune elimination and rejection of biomaterials and utilize biomaterials to induce antigen-specific immune tolerance. There are three research directions in our laboratory: 1) cell membrane-derived materials, 2) long circulating nanoparticles, and 3) biomaterials/biological materials for inducing antigen-specific immune tolerance. These three thrusts are synergized to innovate materials for inducing immune tolerance.
Cell Membrane-derived Materials
Cell membranes carry self-proteins and complement regulators that reduce inflammatory responses to cell membranes and cell membrane-coated materials. We have invented an approach to conjugate peptide, protein, and nanoparticles on cell membranes without affecting cell viability, proliferation or differentiation. Our lab applies this chemical approach to increase the functions of cell membrane-derived materials.
Long Blood Circulating Nanoparticles
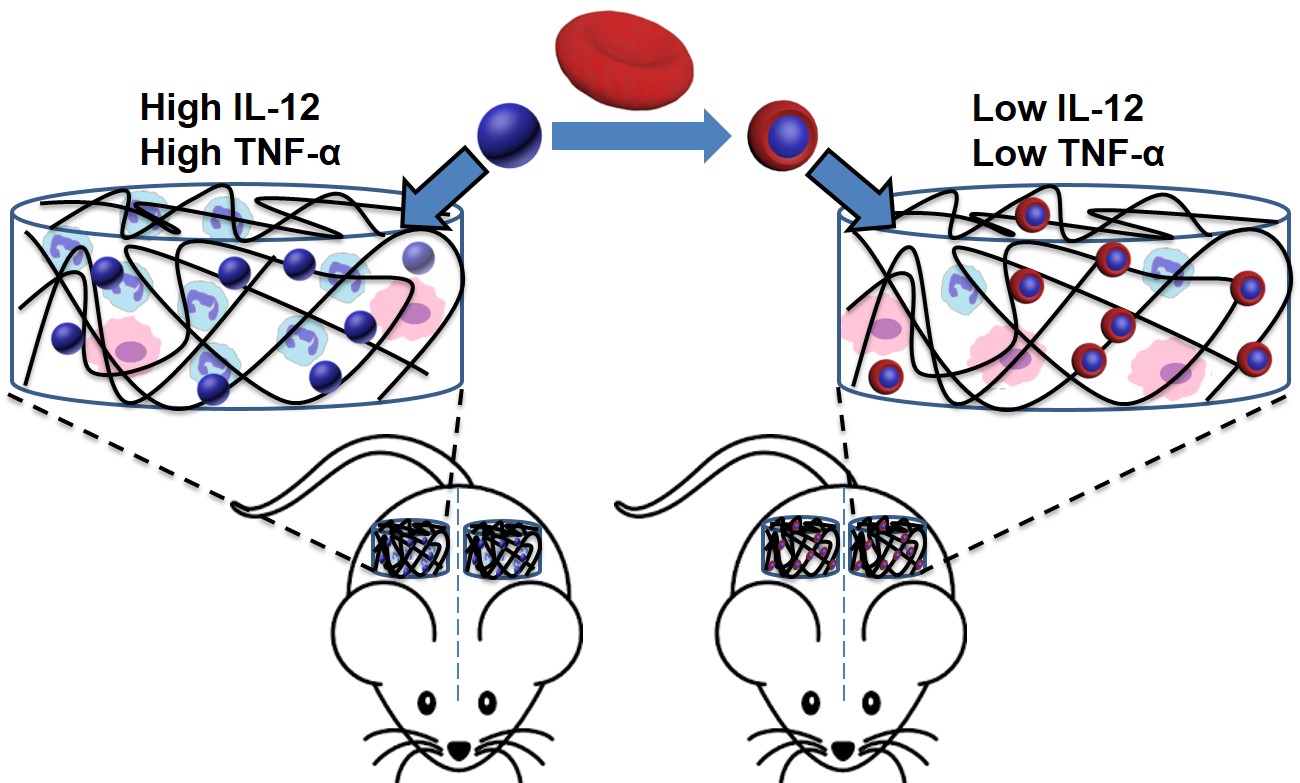
Research into long-circulating nanoparticles has in the past focused on reducing their clearance by macrophages. By engineering a hierarchical polyethylene glycol (PEG) structure on nanoparticle surfaces, we revealed an alternative mechanism to enhance nanoparticle blood circulation. The conjugation of a second PEG layer at a density close to, but lower than the mushroom-to-brush transition regime on conventional PEGylated nanoparticles dramatically prolongs their blood circulation via reduced nanoparticle uptake by non-Kupffer cells in the liver, especially liver sinusoidal endothelial cells (LSECs). Our study also disclosed that the dynamic outer PEG layer reduces protein binding affinity to nanoparticles, although not the total number of adsorbed proteins. These effects of the outer PEG layer diminishes in the higher density regime. Therefore, our results suggest that the dynamic topographical structure of nanoparticles is an important factor in governing their fate in vivo. This facile approach may be used to selectively targeting liver cells.
Biomaterials for Inducing Antigen-Specific Immune Tolerance
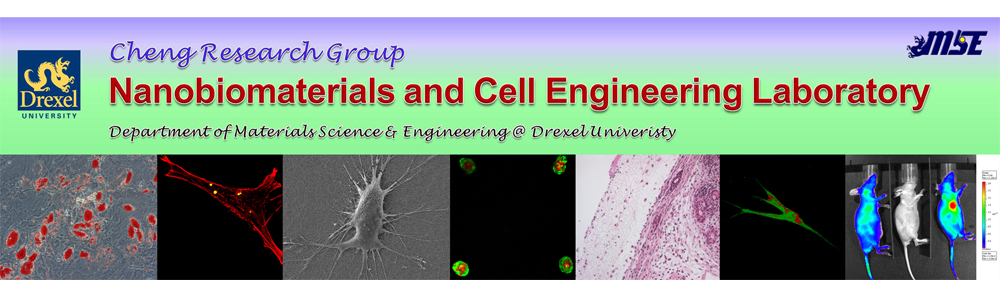
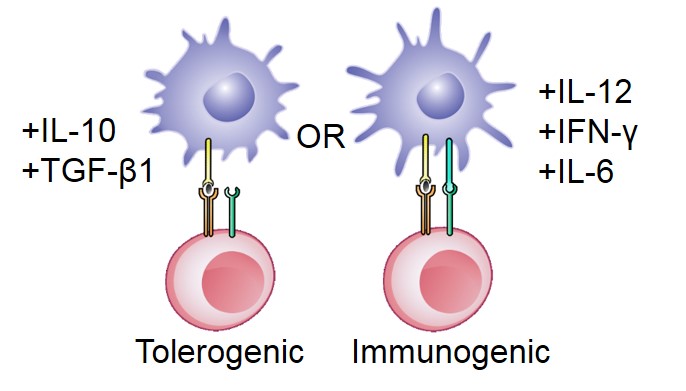
Autoimmune diseases arise from a loss of immunological self-tolerance, and affect over 23.5 million Americans. Current treatment of the majority of these diseases focuses on either treating the symptoms, as in type 1 diabetes, or globally suppressing the immune system, as in multiple sclerosis. Unfortunately, these treatments are usually lifelong, inadequate, and are associated with a range of adverse effects. Therefore, recent research has directed its focus towards inducing antigen-specific tolerance. We are developing biocompatible porous scaffold, containing cytokines, antigen, and tolerogenic molecules for generating tolerogenic dendritic cells. These cells can subsequently migrate to lymphoid or peripheral tissues to tolerize autoreactive T cells and treat systemic autoimmunity.