The Interplay of Aggregation, Fibrillization and Gelation of an Unexpected Low Molecular Weight Gelator: Glycylalanylglycine in Ethanol/Water
Abstract
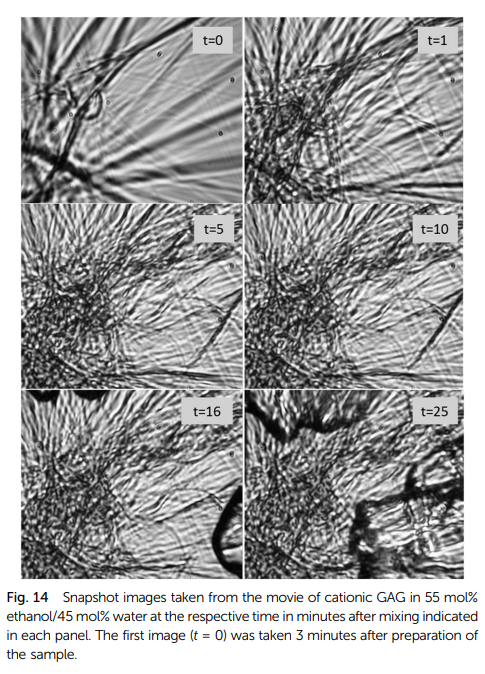
Hydrogels formed by polypeptides could be much-favored tools for drug delivery because their main ingredients are generally biodegradable. However, the gelation of peptides in aqueous solution generally requires a minimal length of the peptide as well as distinct sequences of hydrophilic and hydrophobic residues. The aggregation of short peptides like tripeptides, which are relatively cheap and offer a high degree of biodegradability, are generally thought to require a high hydrophobicity of their residues. We found that contrary to this expectation cationic glycylalanylglycine in 55 mol% ethanol/45 mol% water forms a gel below a melting temperature of ca. 36 °C. A pure hydrogel state can be obtained after allowing the ethanol component to evaporate. The gel phase consists of crystalline fibrils of several 100 μm, which form a sample-spanning network. Rheological data reveal a soft elastic solid gel. We investigated the kinetics of the various processes that lead to the final gel state of the ternary mixture by a unique combination of UV circular dichroism, infrared, vibrational circular dichroism (VCD) and rheological measurements. A mathematical analysis of our data show that gelation is preceded by the formation of peptide β-sheet like tapes or ribbons, which give rise to a significant enhancement of the amide I′ VCD signal, and the subsequent formation of rather thick and long fibrils. The VCD signals indicate that the tapes exhibit a right-handed helicity at temperatures above 16 °C and a left-handed helicity below. The tapes’/ribbons’ helicity change occurs at a temperature where the UVCD data reflect a relatively long nucleation process. The kinetics of gel formation probed by the storage and loss moduli are composed of a fast process that follows tape/ribbon/fibril formation and is clearly identifiable in a movie that shows the gelation process and a slow process that causes an additional gel stabilization. The rheological data indicate that left-handed fibrils observed at low temperatures form a more solid-like structure than their right-handed counterparts formed at higher temperatures. Taken together our data reveal GAG as an unexpected gelator, the formation of which is underlied by a set of distinguishable kinetic processes.
Publication Metadata
Authors
Stefanie Farrell, David DiGuiseppi, Nicolas Alvarez, Reinhard Schweitzer-Stenner
Keywords
Citation
Farrell S, DiGuiseppi D, Alvarez NJ, Schweitzer-Stenner R. The interplay of aggregation, fibrillization and gelation of an unexpected low molecular weight gelator: glycylalanylglycine in ethanol/water. Soft Matter, 2016, 12, 6096-6110. DOI: https://doi.org/10.1039/C6SM00879H
