Investigation of Peptide-based Models for Drug Delivery
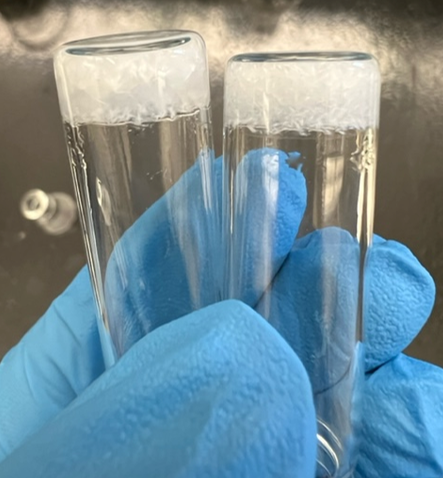
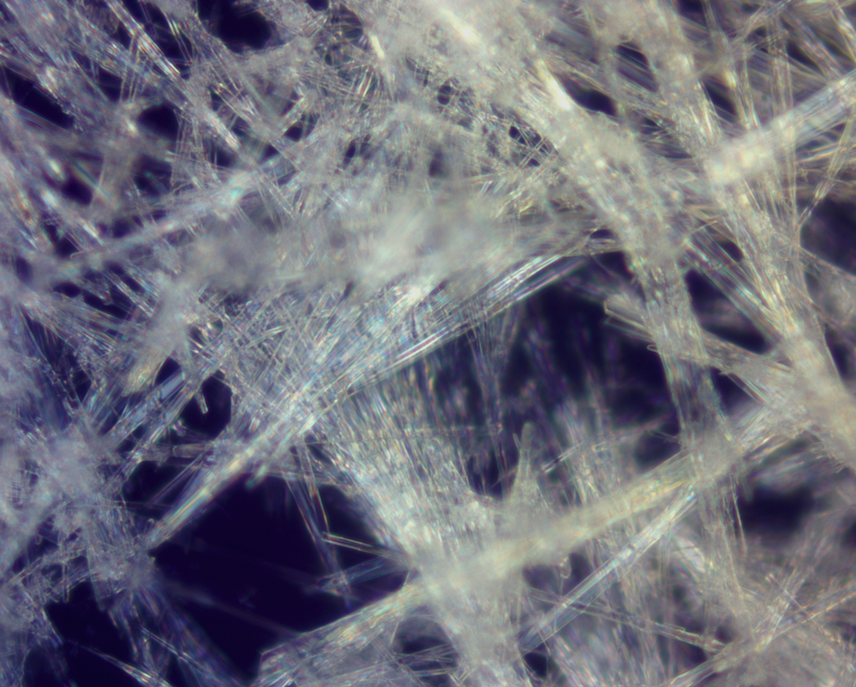

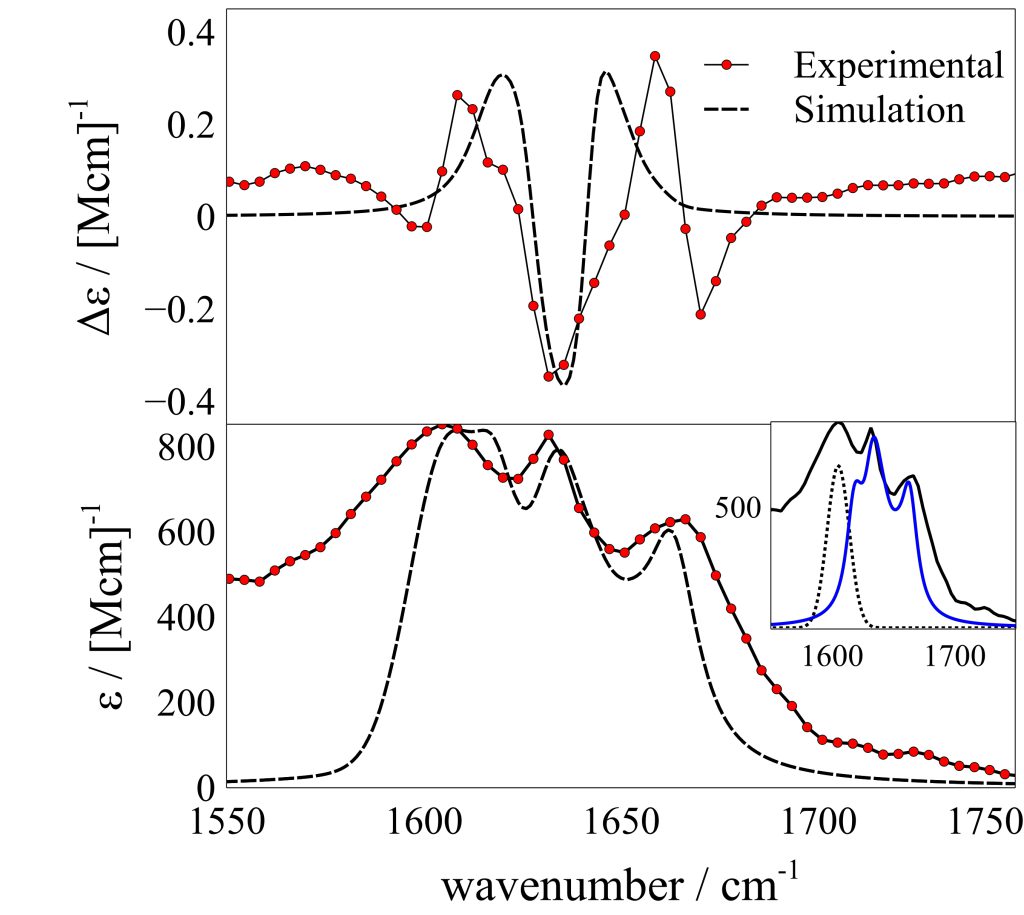
My research involves studying the phenomena of self-assembly of tripeptides that have the general motif GXG, where a variable residue with aromatic properties (X) is flanked on both sides by glycine (G). The nanostructure of the fibrils are investigated using vibrational spectroscopy (FTIR and vibrational circular dichroism) and powder x-ray diffraction. The crystal structures are validated using in-house vibrational spectra programs that simulate the structure sensitive amide I dispersion patterns. We exploit our VCD experimental setup to determine the main axis of the fibril system using the reduced dimensionality of our system. This information gives insight into the fibril-drug interface, which is critical in efforts to developing systems for drug delivery.
Project by

Nichole O'Neill
Project Title
Keywords
Co-Advisor
Networking
[ Research Gate ][ Linkedin ][ Google Scholar ]
Website
Scan or click the link to view more about me and my research.
Research Techniques
vibrational circular dichroism (VCD) spectroscopy, infrared (IR) spectroscopy, Raman spectroscopy, electronic circular dichroism (ECD) spectroscopy, UV/Vis spectroscopy, small amplitude oscillatory shear (SAOS) rheology

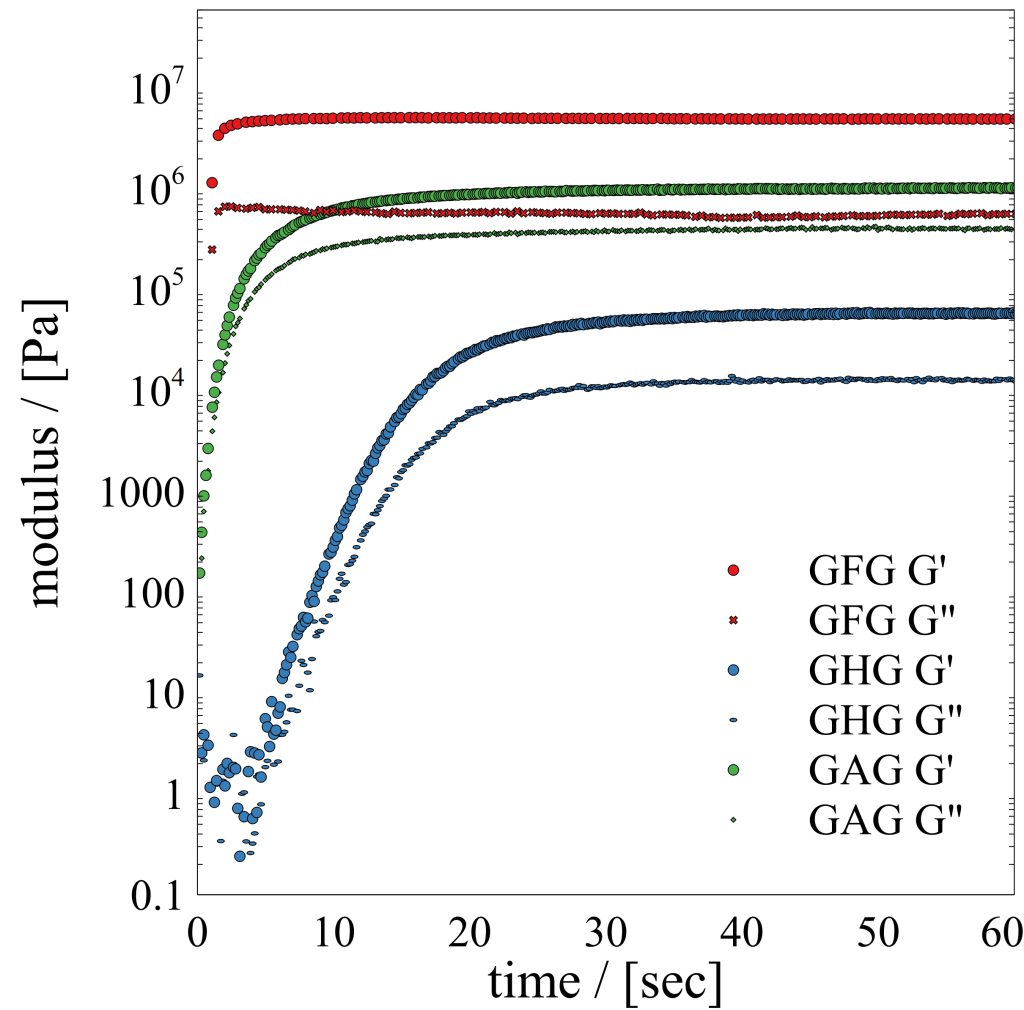
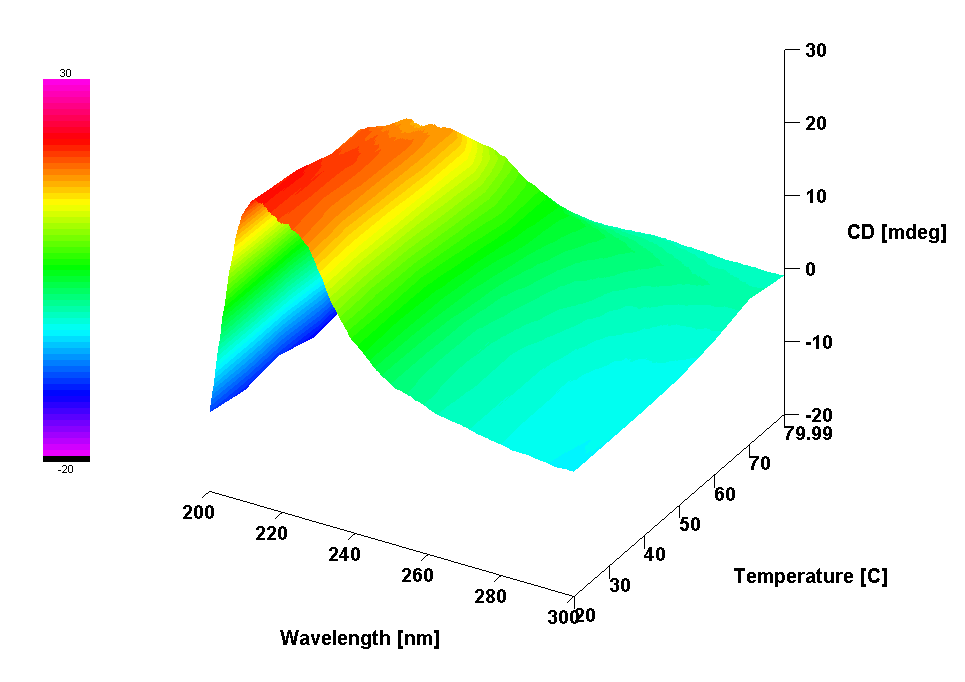
Publications
Schweitzer-stenner R.; Kurbaj, R.; O’Neill, N.; Andrews, B.; Shah, R.; Urbanc, B. Conformational Manifold Sampled by Two Short Linear Motif Segments Probed by Circular Dichroism, Vibrational, and Nuclear Magnetic Resonance Spectroscopy. Biochemistry 2023. https://doi.org/10.1021/acs.biochem.3c00212.
Thursch, L. J.; Lima, T. A.; O’Neill, N.; Ferreira, F. F.; Schweitzer-Stenner, R.; Alvarez, N. J. Influence of Central Sidechain on Self-Assembly of Glycine-x-Glycine Peptides. Soft Matter 2022. https://doi.org/10.1039/D2SM01082H.
O’Neill, N.; Lima, T. A.; Ferreira, F. F.; Thursch, L.; Alvarez, N.; Schweitzer-Stenner, R. Forbidden Secondary Structures Found in Gel-Forming Fibrils of Glycylphenylalanylglycine. J. Phys. Chem. B 2022. https://doi.org/10.1021/acs.jpcb.2c05010.
Milorey, B.; Schwalbe, H.; O’Neill, N.; Schweitzer-Stenner, R. Repeating Aspartic Acid Residues Prefer Turn-like Conformations in the Unfolded State: Implications for Early Protein Folding. J. Phys. Chem. B 2021, 125 (41), 11392–11407. https://doi.org/10.1021/acs.jpcb.1c06472.