The impact of thermal history on the structure of glycylalanylglycine ethanol/water gels
Abstract
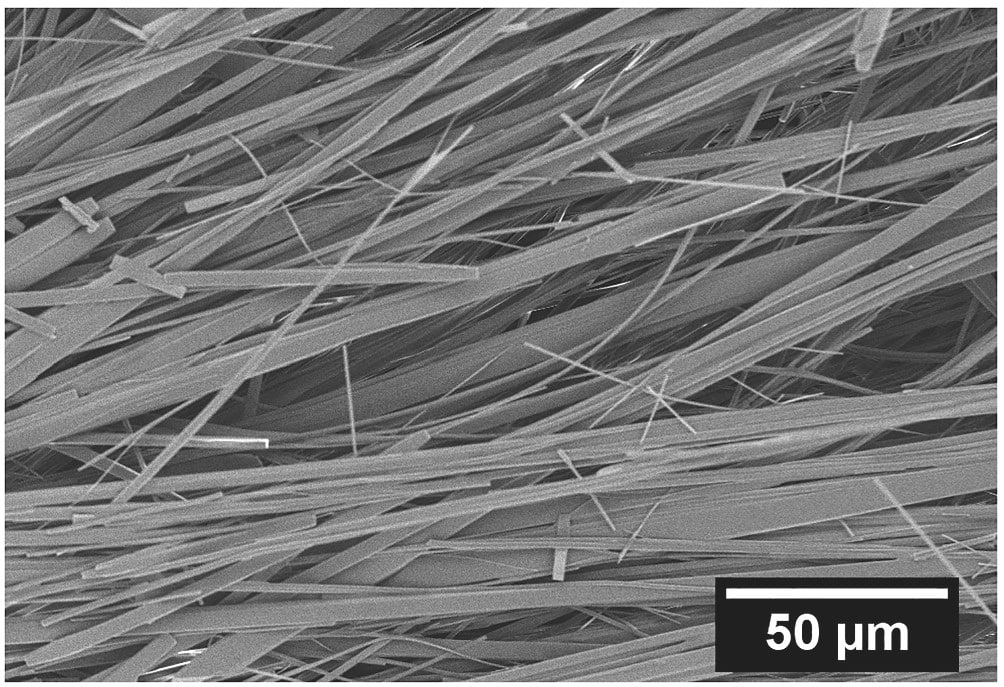
This work revisits several open questions regarding the mechanisms of GAG fibril formation and structure as a function of temperature. The authors recently hypothesized that there is a solubility limit of GAG in ethanol/water that induces self-assembly. In other words, not all peptides can participate in fibrillization and some fraction is still soluble in solution. We show via FTIR spectroscopy that, indeed, free peptides are still present in solution after fibril formation, strongly supporting the solubility model. Furthermore, previous work showed GAG self-assembled into right-handed (phase I) or left-handed (phase II) chiral structures depending on temperature. In this study, we analyze the crystalline structure of phase I and II gels via WAXS and SAXS to compare their crystalline structures and order. Rheological measurements were used to investigate the response of the fibrillar network to temperature. They reveal that the ability of the peptide to self-assemble depends on the solubility at a given temperature and not on thermal history. Furthermore, the gel softening point, the linear viscoelastic gel microstructure, and relaxation spectrum are very similar between phase I and phase II. Overall, the temperature only affects the chirality of the fibrils and the formation kinetics.
Publication Metadata
Authors
Lavenia J. Thursch, Thamires A. Lima, Reinhard Schweitzer-Stenner, Nicolas J. Alvarez
Keywords
DOI
Citation
Thursch, LJ, Lima, TA, Schweitzer-Stenner, R, Alvarez, NJ. The impact of thermal history on the structure of glycylalanylglycine ethanol/water gels. J Pep Sci. 2021; 27:e3305. https://doi.org/10.1002/psc.3305
