Research
The research in our laboratory is focused on the development and characterization of novel nanostructured materials, systems and architectures for batteries, supercapacitors and fuel cells in order to understand electrochemical energy storage and conversion at nanoscale. This fundamental knowledge will help to address practical issues and develop new electrochemical systems for efficient sustainable energy technology. In particular, we are interested in improving the performance characteristics of lithium-ion batteries through fabrication of nano/mesoscale composite electrode materials and solid-state electrolytes. We also explore emerging technologies for energy storage applications, such as metal-air batteries, hybrid supercapacitors, and multivalent intercalation systems, and carry out research to contribute in these directions. In addition, we work on developing new approaches for the integration of nanomaterials into devices to enable system-level characterization and to meet the need for long stability and high performance.
Electrochemical Systems
Beyond Lithium Ion Batteries
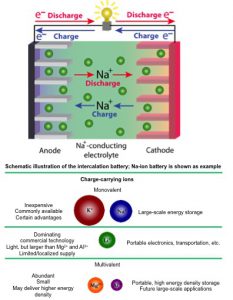 Continuous developments in technology lead to the quick growth of both the number of applications requiring autonomous power and size of the energy storage devices. Because of their light weight and highly electropositive nature, lithium ions are likely to be used as charge carriers for at least the next few years. However, future affordable energy storage solutions could utilize less expensive electrode materials and so called “beyond lithium ions” (BLIs: Na+, K+, Mg2+, Zn2+, Al3+, etc.) in electrolytes. Our focus on Beyond Lithium Ion Batteries is motivated by the global need to identify alternate intercalation chemistries that can alleviate the supply and geographic constraints associated with sourcing the lithium for Li-ion batteries, particularly for large-format storage. Although heavier than lithium, in addition to their lower price, these ions have other advantages. For example, larger monovalent ions (Na+ and K+) require lower desolvation energy than the smaller Li+ ion, thus improving the kinetics of the ion insertion process at the electrode/electrolyte interface, which is important for high power. In addition, unlike lithium, sodium does not form an alloy with aluminum. Therefore, heavy and expensive copper current collectors can be replaced with aluminum for anodes in Na-ion batteries. At the same time, for applications where volumetric performance plays a key role, such as wearables and small electronics, high energy density in a small volume can be achieved by combining the best 2D electrode materials with multivalent ions (Mg2+ and Al3+).
Continuous developments in technology lead to the quick growth of both the number of applications requiring autonomous power and size of the energy storage devices. Because of their light weight and highly electropositive nature, lithium ions are likely to be used as charge carriers for at least the next few years. However, future affordable energy storage solutions could utilize less expensive electrode materials and so called “beyond lithium ions” (BLIs: Na+, K+, Mg2+, Zn2+, Al3+, etc.) in electrolytes. Our focus on Beyond Lithium Ion Batteries is motivated by the global need to identify alternate intercalation chemistries that can alleviate the supply and geographic constraints associated with sourcing the lithium for Li-ion batteries, particularly for large-format storage. Although heavier than lithium, in addition to their lower price, these ions have other advantages. For example, larger monovalent ions (Na+ and K+) require lower desolvation energy than the smaller Li+ ion, thus improving the kinetics of the ion insertion process at the electrode/electrolyte interface, which is important for high power. In addition, unlike lithium, sodium does not form an alloy with aluminum. Therefore, heavy and expensive copper current collectors can be replaced with aluminum for anodes in Na-ion batteries. At the same time, for applications where volumetric performance plays a key role, such as wearables and small electronics, high energy density in a small volume can be achieved by combining the best 2D electrode materials with multivalent ions (Mg2+ and Al3+).
However, beyond lithium ions are either larger in size than lithium ion, or they carry a higher charge, which limits their diffusion in the crystal lattice of electrode materials. In addition, Na-ion batteries (SIBs) and Mg-ion batteries (MIBs) operate at lower voltages than Li-ion batteries, and thus higher capacity cathode materials are necessary to increase the energy storage of SIBs and MIBs. In the MEG laboratory, we design, synthesize, characterize and test new electrode materials for BLI batteries with the aim to achieve record high electrochemical performance in these emerging energy storage systems.
Capacitive Water Deionization
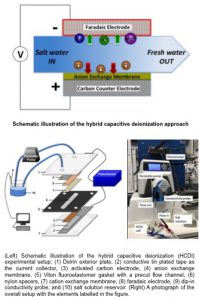 Clean water is essential for life, but attaining affordable, fresh, and uncontaminated water still remains a grand challenge. Only ~0.3% of the water present on Earth is freshwater in the liquid form on the surface, while remaining freshwater resources exist in the form of glaciers/ice and groundwater. As a result, it is of great interest to develop cost-effective and high efficiency approaches to desalinate the large supply of brackish or salty water available on our planet. Currently, the most common methods used to desalinate water are reverse osmosis, electrodialysis, and thermal separation, but these processes require high operating cost and/or significant energy inputs. Capacitive deionization (CDI) represents a less expensive and more energy efficient technology that does not produce secondary pollution and allows for easy regeneration and maintenance. CDI functions via charge storage mechanism analogous to supercapacitors. In this process, saline water flows by or through two electrode materials, while a potential is applied across these electrodes. In this first step, ion electrosorption, ions are extracted out of the water and absorbed into the structure of the electrodes, resulting in desalinated water that exits from the system. On the subsequent step, ion desorption, the potential is brought to zero or a negative potential is applied, forcing ions out of the structure of the electrodes to be washed from the system and thus regenerating the electrodes for the next ion electrosorption step.
Clean water is essential for life, but attaining affordable, fresh, and uncontaminated water still remains a grand challenge. Only ~0.3% of the water present on Earth is freshwater in the liquid form on the surface, while remaining freshwater resources exist in the form of glaciers/ice and groundwater. As a result, it is of great interest to develop cost-effective and high efficiency approaches to desalinate the large supply of brackish or salty water available on our planet. Currently, the most common methods used to desalinate water are reverse osmosis, electrodialysis, and thermal separation, but these processes require high operating cost and/or significant energy inputs. Capacitive deionization (CDI) represents a less expensive and more energy efficient technology that does not produce secondary pollution and allows for easy regeneration and maintenance. CDI functions via charge storage mechanism analogous to supercapacitors. In this process, saline water flows by or through two electrode materials, while a potential is applied across these electrodes. In this first step, ion electrosorption, ions are extracted out of the water and absorbed into the structure of the electrodes, resulting in desalinated water that exits from the system. On the subsequent step, ion desorption, the potential is brought to zero or a negative potential is applied, forcing ions out of the structure of the electrodes to be washed from the system and thus regenerating the electrodes for the next ion electrosorption step.
The performance of a CDI system to the large extend depends on two parameters: (1) the choice of electrode material and (2) the electrode architecture. In the MEG laboratory, we focus on designing, synthesizing and exploring water desalination properties of the faradaic electrode materials in hybrid capacitive deionization system. This type of system takes advantage of the ability of the faradaic electrode to undergo redox reactions with cations (analogous to the reactions occurring at the cathode in a battery), thus removing them from solution. This type of electrode has the potential to achieve much higher desalination capacities compared to the surface-based ion adsorption exhibited by carbon electrodes. In addition, we assemble high performing materials to construct electrodes with highly porous architecture and good transport of both electrons and ions to maximize performance characteristics.
Materials
Tunnel Manganese Oxides
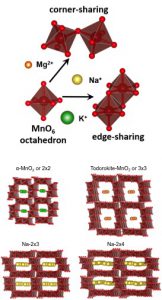 The choice of manganese oxide as the active electrode material is motivated by its low cost, abundance of manganese, and its environmental friendliness. In addition, manganese oxides exhibit rich crystallography forming various electrochemically active crystal structures. Importantly, it is stable in contact with both non-aqueous and aqueous solutions. Also, the ability of manganese to reduce from Mn4+ to Mn3+ facilitates interaction with positively charged ions through surface reactions and volume intercalation, opening a way to explore performance of manganese oxides as both battery and faradaic electrodes for CDI.
The choice of manganese oxide as the active electrode material is motivated by its low cost, abundance of manganese, and its environmental friendliness. In addition, manganese oxides exhibit rich crystallography forming various electrochemically active crystal structures. Importantly, it is stable in contact with both non-aqueous and aqueous solutions. Also, the ability of manganese to reduce from Mn4+ to Mn3+ facilitates interaction with positively charged ions through surface reactions and volume intercalation, opening a way to explore performance of manganese oxides as both battery and faradaic electrodes for CDI.
In the MEG laboratory, we are particularly interested in manganese oxides with tunnel crystal structures, a special materials family displaying a controlled variation in tunnel size and shape which can be used for ion intercalation. Tunnel manganese oxides are built by MnO6 octahedra sharing corners and edges. Different combinations of the octahedral stacking can be achieved by carefully controlling synthesis parameters. The tunnel dimensions and shape depend on the radius and amount of so called stabilizing or templating ions added to a precursor mixture during hydrothermal synthesis process. Stabilizing ions reside in side the tunnel space in formed materials. Manganese oxides with the stabilized structure have a general formula of AxMnO2 (A = Na, Mg, K, etc.). From the crystal structure point of view, they can be divided into two categories: (1) structures with rectangular tunnel shape and (2) structures with non-rectangular tunnel shape. The rectangular tunnels are formed by MnO6 octahedra sharing edges on each side of the rectangle; the rectangle sides are connected by octahedra sharing corners. The structures are named by indicating the number of octahedra on the perpendicular rectangle sides, for example, 2×2 or 2×3, etc. We focus on the following manganese oxide phases with the rectangular tunnels: α-MnO2 or 2×2; 2×3; 2×4; and todorokite or 3×3.
Layered Oxides
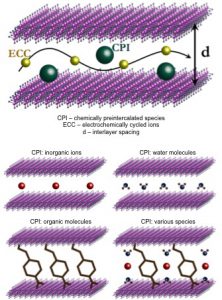 Metastable layered insertion compounds offer an expanded space for new intercalation-related phenomena and enhanced functionalities that cannot be achieved by compounds at thermodynamic equilibrium. They have stimulated intense interest in their use in applications that involve electrochemically driven movement of inorganic ions through well-defined two-dimensional (2D) diffusion channels, ranging from energy storage and electrochromics to sensing and actuation to water treatment. Due to the relatively high working voltages, in energy storage transition metal oxides are considered to be especially attractive candidates for cathode applications.
Metastable layered insertion compounds offer an expanded space for new intercalation-related phenomena and enhanced functionalities that cannot be achieved by compounds at thermodynamic equilibrium. They have stimulated intense interest in their use in applications that involve electrochemically driven movement of inorganic ions through well-defined two-dimensional (2D) diffusion channels, ranging from energy storage and electrochromics to sensing and actuation to water treatment. Due to the relatively high working voltages, in energy storage transition metal oxides are considered to be especially attractive candidates for cathode applications.
In the MEG laboratory, we design, synthesize, characterize and test novel metastable layered insertion oxides, which are not accessible by conventional high-temperature synthesis techniques, with large controlled interlayer spacing, enabling an increased number of intercalation sites and facilitated diffusion of the electrochemically cycled ions. We are especially interested in oxides of transition metals in high oxidation states, such as vanadium, molybdenum and tungsten oxides, that can undergo several reduction steps accompanied by the transfer of several electrons, which leads to a high capacity. Additionally, we develop methods to control chemistry and structure of the metastable layered oxides through introducing interlayer species, such as inorganic ions, water molecules, and organic molecules. By tuning the nature and amount of the interlayer species, we investigate their effect on the electrochemical properties of the synthesized new materials.
Synthesis Approaches
Hydothermal Treatment
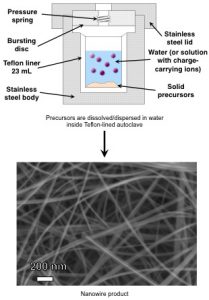 Hydrothermal treatment is a solution based synthesis approach that yields large quantities of nanostructured materials and provides robust control over the growth process to tailor the dimensions, composition and structure of the nanomaterials. Typically, solid and aqueous precursors are added to a Teflon-liner and placed in a stainless steel autoclave that is sealed and treated at elevated temperatures.
Hydrothermal treatment is a solution based synthesis approach that yields large quantities of nanostructured materials and provides robust control over the growth process to tailor the dimensions, composition and structure of the nanomaterials. Typically, solid and aqueous precursors are added to a Teflon-liner and placed in a stainless steel autoclave that is sealed and treated at elevated temperatures.
In the MEG laboratory, we are particularly interested in using high pressure and high temperature conditions inside of the autoclave to produce highly crystalline nanoscale materials with high aspect ratio, one-dimensional morphology that can be challenging to obtain through other synthesis methods. Material morphology, composition, and crystal structure can be carefully tailored by controlling the treatment temperature, volume of solution in the autoclave, and the chemical composition/amount of the solid and aqueous precursors. Thus, this method provides an ideal platform for carefully controlling the properties of nanomaterials in order to study the structure-property-performance relationships of novel nanomaterials in emerging electrochemical energy and water applications.
Chemical Pre-Intercalation
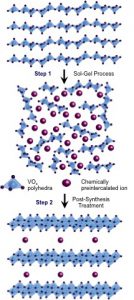 Many transition metal oxides can be synthesized through sol-gel processes, forming phases that are electrochemically active in reversible ion cycling reactions. The classic sol-gel process has the distinct advantage of producing solid materials from chemically homogeneous solutions. Due to the atomic-level mixing, sol-gel chemistry offers great control over material composition and structure. Moreover, lower processing temperatures allow us to synthesize metastable phases that cannot be obtained by conventional high-temperature synthesis methods.
Many transition metal oxides can be synthesized through sol-gel processes, forming phases that are electrochemically active in reversible ion cycling reactions. The classic sol-gel process has the distinct advantage of producing solid materials from chemically homogeneous solutions. Due to the atomic-level mixing, sol-gel chemistry offers great control over material composition and structure. Moreover, lower processing temperatures allow us to synthesize metastable phases that cannot be obtained by conventional high-temperature synthesis methods.
Taking advantage of this wet chemistry process, in the MEG laboratory, we developed a chemichal pre-intercalation synthesis process, in which specific types and amounts of guest species, such as inorganic ions and inorganic/organic molecules, are inserted into the crystal structure of host materials that have high redox activity such as transition metal oxides, leading to unprecedented materials diversity. During sol-gel step, chemically preintercalated species are added into the reaction mixture, directing formation of novel metastable layered structures with large controllable interlayer spacings. These spacings provide distinct pathways for diffusion of the electrochemically cycled ions. We also explore the evolution of materials composition and structure during post-synthesis processes, such as aging, hydrothermal treatment and annealing, as well as effects of electrochemical cycling, including transformations that occur during intercalation of the electrochemically cycled ions in the single cycle and long-term transformation of the materials over multiple cycles. By characterizing chemically pre-intercalated phases, important structure–property correlations in this class of metastable insertion oxides can be derived. The insight that is gained from transition metal oxides can be applied to non-oxide hosts, such as transition metal carbides (MXenes) and dichalogenides, to demonstrate the broader potential of this synthesis strategy to a wide range of insertion compounds.